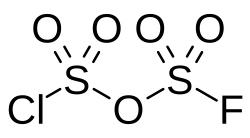
Disulfuryl chloride fluoride
 | |
| Names | |
|---|---|
| Preferred IUPAC name
[(fluorosulfonyl)oxy]sulfonyl chloride | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| ClFO5S2 | |
| Molar mass | 198.56 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.7934 g/cm3 |
| Boiling point | 100 °C (212 °F; 373 K) |
| reacts with water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Disulfuryl chloride fluoride (pyrosulfuryl chloride fluoride) is an inorganic compound of sulfur, chlorine, fluorine, and oxygen with the chemical formula S2O5ClF.[2] Structurally, it is the chlorofluorosulfuric acid analog of disulfuric acid, or the mixed anhydride of chlorosulfuric acid and fluorosulfuric acid.
Synthesis
Rapid heating of fluorosulfonic acid and cyanuric chloride:[3]
- 6HSO3F + (CNCl)3 → 3S2O5ClF + (HNCO)3 + 3HF
Also, a reaction of disulfuryl chloride (S2O5Cl2) on silver monofluoride (AgF) at 80-90 °C.[4]
Physical properties
Disulfuryl chloride fluoride is a colorless liquid.
The compound hydrolyzes in water.
See also
References
- ^ "Disulfuryl chloride fluoride | Chemical Substance Information | J-GLOBAL". J-GLOBAL. Retrieved 18 August 2025.
- ^ Dykyj, J.; Svoboda, J.; Wilhoit, R. C.; Frenkel, M. L.; Hall, K. R. (1999). "Vapor Pressure of Chemicals: Part A. Vapor Pressure and Antoine Constants for Hydrocarbons and Sulfur, Selenium, Tellurium and Hydrogen Containing Organic Compounds". NIST. Retrieved 18 August 2025.
- ^ Inorganic Syntheses: Volume XI. New York: John Wiley & Sons. 2009. p. 153. ISBN 9780470132753. Retrieved 18 August 2025.
- ^ Simons, J. H. (2 December 2012). Fluorine Chemistry V5. Elsevier. p. 71. ISBN 978-0-323-14724-8. Retrieved 20 August 2025.