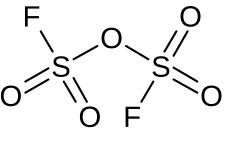
Disulfuryl fluoride
 | |
| Names | |
|---|---|
| Other names
Pyrosulfuryl fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.624 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F2O5S2 | |
| Molar mass | 182.11 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.75 g/cm3 |
| Melting point | –48 °C |
| Boiling point | 50.8 °C |
| reacts with water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Disulfuryl fluoride is an inorganic compound of sulfur, fluorine, and oxygen with the chemical formula S2O5F2.
Synthesis
Autoclave heating of sulfur trioxide and calcium fluoride:
- 2SO3 + CaF2 → S2O5F2 + CaSO4
The compound can be produced by the reaction of SSF2 and FOSO2F in deuterated chloroform at −100 °C:[1]
- 2SSF2 + 4FOSO2F → 2S2O5F2 + 2SOF2 + SF4 + 1⁄8S8
It can also be produced in the reaction of iodine pentafluoride and sulfur trioxide.[2]
Other methods are also known.[3]
Physical properties
Pyrosulfuryl difluoride forms a colorless liquid that smokes slightly in the air. It causes severe suffocation and resembles phosgene in its action.[4][5]
Chemical properties
When heated, the compound is stable to a temperature of 200 °C.
It is slowly hydrolyzed by water:
- S2O5F2 + H2O → 2HSO3F
It reacts with tetraethyl titanate to produce diethoxytitanium difluorosulfonate:[6]
- Ti(OC2H5)4 + 2S2O5F2 → Ti(OC2H5)2(O3SF)2 + 2C2H5OSO2F
See also
References
- ^ Willner, H.; Mistry, F.; Aubke, F. (1 December 1992). "Selected reactions of fluorine-fluorosulfate, FOSO2F". Journal of Fluorine Chemistry. 59 (3): 333–349. doi:10.1016/S0022-1139(00)80329-2. ISSN 0022-1139. Retrieved 15 August 2025.
- ^ Cernik, M. (1987). "ChemInform Abstract: Reaction of Iodine Pentafluoride with Sulfur Trioxide". ChemInform. 18 (33) chin.198733027. doi:10.1002/chin.198733027. ISSN 1522-2667. Retrieved 15 August 2025.
- ^ Inorganic Syntheses, Volume 11. John Wiley & Sons. 22 September 2009. p. 151. ISBN 978-0-470-13277-7. Retrieved 16 August 2025.
- ^ Simons, J. H. (2 December 2012). Fluorine Chemistry V5. Elsevier. p. 70. ISBN 978-0-323-14724-8. Retrieved 16 August 2025.
- ^ Ryss, Iosif Grigorʹevich (1960). The Chemistry of Fluorine and Its Inorganic Compounds. U. S. Atomic Energy Commission, Technical Information Service Extension. p. 185. Retrieved 16 August 2025.
- ^ Niyogi, Debyani G.; Singh, Sukhjinder; Saini, Anju; Verma, R. D. (1 February 1994). "Reactions of fluorinated acid anhydrides with metal alkoxides". Journal of Fluorine Chemistry. 66 (2): 153–158. Bibcode:1994JFluC..66..153N. doi:10.1016/0022-1139(93)03012-B. ISSN 0022-1139. Retrieved 15 August 2025.