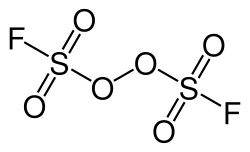
Peroxydisulfuryl difluoride
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F2O6S2 | |
| Molar mass | 198.11 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.645 g/cm3 |
| Melting point | −55.4 °C |
| Boiling point | 67.1 °C |
| reacts with water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Peroxydisulfuryl difluoride is an inorganic compound from the group of peroxides.[1] The chemical formula is F2O6S2.
Synthesis
Peroxydisulfuryl difluoride can be obtained by the reaction of sulfur trioxide with fluorine in the presence of silver(II) fluoride or by electrolysis of fluorosulfuric acid.[2]
- 2SO3 + F2 → S2O6F2
It is also possible to prepare it by reacting chromium(V) fluoride with sulfur trioxide:[3]
- CrF5 + 5SO3 → S2O6F2 + Cr(SO3F)3
or by the reaction between fluorosulfuric acid and dioxygenyl hexafluoroarsenate:[4]
- 2HSO3F + 2[O2][AsF6] → S2O6F2 + 2O2 + 2AsF5 + 2HF
Physical properties
Peroxydisulfuryl difluoride is a colorless liquid with an unpleasant odor that hydrolyzes with water to produce oxygen and fluorosulfuric acid. The compound can ignite organic materials upon contact.[2]
Chemical properties
It reacts with cesium fluorosulfonate and silver fluorosulfonate to produce the divalent silver compound CsAg(SO3F)3.[5]
Iodine(I) fluorosulfonate can be obtained from iodine and peroxydisulfuryl difluoride:[6]
- I2 + S2O6F2 → 2ISO3F
Uses
Peroxydisulfuryl difluoride can be used to produce fluorosulfates. It is a strong oxidizing agent and can be used for the oxidation of noble metals (Ag Au Re Pt Os Rh) to the salts of fluorosulfuric acid.[2]
See also
References
- ^ Dudley, F. B.; Cady, G. H. (1 February 1957). "Peroxydisulfuryl Difluoride". Journal of the American Chemical Society. 79 (3): 513–514. Bibcode:1957JAChS..79..513D. doi:10.1021/ja01560a002. ISSN 0002-7863. Retrieved 13 August 2025.
- ^ a b c Zefirov, Nikolai S. (2001). "Peroxydisulfuryl Difluoride". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289x.rp044. ISBN 0-471-93623-5. Retrieved 13 August 2025.
- ^ Brown, S. D.; Gard, G. L. (1 January 1975). "A new preparation of peroxydisulfuryl difluoride". Inorganic and Nuclear Chemistry Letters. 11 (1): 19–21. doi:10.1016/0020-1650(75)80140-1. ISSN 0020-1650. Retrieved 13 August 2025.
- ^ Šmalc, Andrej; Mayorga, Steven G.; Bartlett, Neil (1992). "Peroxydisulfuryl Difluoride (Modification)". Inorganic Syntheses. Inorganic Syntheses. Vol. 29. John Wiley & Sons, Ltd. pp. 10–11. doi:10.1002/9780470132609.ch5. ISBN 978-0-471-54470-8. Retrieved 13 August 2025.
- ^ Michałowski, T.; Mazej, Z.; Budzianowski, A.; Jagličić, Z.; Leszczyński, P. J.; Grochala, W. (2015). "Unexpectedly Complex Crystalline Phases in the MSO3F–Ag(SO3F)2 Phase Diagram (M = Na, K, Rb, Cs)". European Journal of Inorganic Chemistry. 2015 (2): 324–332. Bibcode:2015EJIC.2015..324M. doi:10.1002/ejic.201402948. ISSN 1099-0682. Retrieved 13 August 2025.
- ^ Cotton, F. Albert (17 September 2009). Progress in Inorganic Chemistry, Volume 7. John Wiley & Sons. p. 65. ISBN 978-0-470-16658-1. Retrieved 14 August 2025.