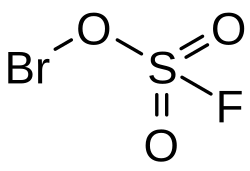
Bromine(I) fluorosulfonate
 | |
| Names | |
|---|---|
| Other names
Bromine(I) fluorosulfate[1], bromine fluorosulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| BrFO3S | |
| Molar mass | 178.96 g·mol−1 |
| Appearance | blackish-red liquid |
| Density | 2.60 g/cm3 |
| Boiling point | 120.5 °C |
| reacts with water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Bromine(I) fluorosulfonate is an inorganic compound of bromine, sulfur, fluorine, and oxygen with the chemical formula BrSO3F. This is a monovalent compound of bromine from the group of fluorosulfonates.
Synthesis
Similarly with other halogenofluorosulfonates, the reaction of bromine with peroxydisulfuryl difluoride produces the compound:[2][3]
- Br2 + S2O6F2 → 2BrSO3F
The reduction of bromine(III) fluorosulfonate also yields bromine(I) fluorosulfonate:[4]
- Br(SO3F)3 + Br2 → 3BrSO3F
Chemical properties
Bromo(I) fluorosulfonate is a blackish-red, viscous, hydrolysis-sensitive liquid that reacts violently with water. Upon cooling, it solidifies into a glassy state.
Bromo(I) fluorosulfonate reacts with iodine(I) fluorosulfonate at temperatures above 50 °C to form dibromoiodofluorosulfonate:[5]
- 2BrSO3F + ISO3F → IBr2(SO3F)3
See also
- Iodine fluorosulfate
- Fluorine fluorosulfate
- Chlorine fluorosulfate
- Triiodine fluorosulfate
References
- ^ Earl, Boyd L.; Hill, Brian K.; Shreeve, Jean'ne M. (1 December 1966). "Reactions of Bromine(I) Fluorosulfate with Simple Inorganic Molecules and Polyfluoroolefins. A Novel Route to Perfluoro-2,3-butanedione". Inorganic Chemistry. 5 (12): 2184–2186. doi:10.1021/ic50046a024. ISSN 0020-1669. Retrieved 18 August 2025.
- ^ Cotton, F. Albert (2009). Progress in Inorganic Chemistry: Volume 14. New York: John Wiley & Sons. p. 65. ISBN 978-0-470-16658-1. Retrieved 14 August 2025.
- ^ Brauer, Georg (1975). Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I (PDF) (3., umgearb. Aufl ed.). Stuttgart: Ferdinand Enke. p. 339. ISBN 3-432-02328-6. Retrieved 14 August 2025.
- ^ Roberts, John E.; Cady, George H. (1 January 1960). "Halogen Fluorosulfonates BrSO3F, Br(SO3F)3 and I(SO3F)3". Journal of the American Chemical Society. 82 (2): 352–353. Bibcode:1960JAChS..82..352R. doi:10.1021/ja01487a023. ISSN 0002-7863. Retrieved 14 August 2025.
- ^ Niedenzu, Kurt; Zimmer, Hans (2013). Annual Reports in Inorganic and General Syntheses (PDF). Academic Press. p. 109. ISBN 978-1-4832-1388-0. Retrieved 14 August 2025.