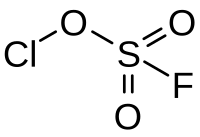
Chlorine fluorosulfate
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| ClFO3S | |
| Molar mass | 134.51 g·mol−1 |
| Appearance | Light yellow liquid |
| Density | 1.71 g/cm³ |
| Melting point | −84.3 °C |
| Boiling point | 43.4 °C |
| reacts with water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Chlorine fluorosulfate is an inorganic compound with the chemical formula ClFO3S.[1] This is a derivative of fluorosulfonic acid.
Synthesis
Chlorine fluorosulfonate can be prepared by reacting sulfur trioxide and chlorine monofluoride at low temperatures:[2]
- SO3 + ClF → ClOSO2F
The compound can also be prepared by reacting sulfonyl fluoride peroxide with chlorine at 125 °C under high pressure:
- Cl2 + S2O6F2 → 2ClOSO2F
Physical properties
The compound is a highly reactive, and forms a pale yellow liquid that reacts violently with water.[3] The compound decomposes upon warming to room temperature, turning red.
Chemical properties
Chlorine trifluoride oxide reacts with chlorine fluorosulfate:
- ClOF3 + 2ClOSO2F → S2O5F2 + FClO2 + 2ClF
The reaction also produces SO2F2.[4]
The compound also reacts with nitronium perchlorate to produce chlorine perchlorate:[5]
- 2ClOSO2F + NO2ClO4 → ClClO4 + NO2SO3F
Uses
The compound is used as a chlorinating agent, fluorosulfating agent, and oxidizer.[6]
See also
- Bromine fluorosulfate
- Fluorine fluorosulfate
- Iodine fluorosulfate
- Triiodine fluorosulfate
References
- ^ Aubke, Friedhelm; Casper, Bernd; Müller, Holger S. P.; Oberhammer, Heinz; Willner, Helge (15 February 1995). "Vibrational spectra and gas phase structures of fluorine fluorosulfate (FOSO2F) and chlorine fluorosulfate (ClOSO2F)". Journal of Molecular Structure. 346: 111–120. doi:10.1016/0022-2860(94)08421-D. ISSN 0022-2860. Retrieved 9 August 2025.
- ^ Hardin, C. V.; Ratcliffe, Charles T.; Anderson, Lowell Ray; Fox, William B. (1 August 1970). "Preparing chlorine fluorosulfate". Inorganic Chemistry. 9 (8): 1938–1939. doi:10.1021/ic50090a035. ISSN 0020-1669. Retrieved 9 August 2025.
- ^ Inorganic Syntheses. John Wiley & Sons. 22 September 2009. p. 8. ISBN 978-0-470-13290-6. Retrieved 9 August 2025.
- ^ Advances in Inorganic Chemistry and Radiochemistry. Academic Press. 9 July 1976. pp. 331–333. ISBN 978-0-08-057867-5. Retrieved 9 August 2025.
- ^ Steudel, Ralf (20 April 2011). Chemistry of the Non-Metals: With an Introduction to Atomic Structure and Chemical Bonding. Walter de Gruyter. p. 266. ISBN 978-3-11-083082-8. Retrieved 9 August 2025.
- ^ Schack, Carl J.; Wilson, Richard D.; Totsch, W. (1986). "Chlorine Fluorosulfate". Inorganic Syntheses. John Wiley & Sons, Ltd. pp. 6–8. doi:10.1002/9780470132555.ch3. ISBN 978-0-470-13255-5. Retrieved 9 August 2025.