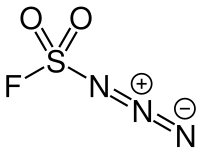
Fluorosulfonyl azide
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| FN3O2S | |
| Molar mass | 125.08 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Fluorosulfonyl azide is an inorganic compound with the chemical formula FN3O2S. This is a derivative of sulfuric acid containing a fluorine atom and an azide group. The compound is used as a reagent for transferring diazo groups.[1]
Synthesis
Fluorosulfonyl azide can be prepared by reacting disulfuryl fluoride (F2S2O5) with sodium azide in nitromethane or sulfolane.[2]
Chemical properties
Fluorosulfonyl azide is a diazo group transfer reagent.[3] It is suitable, for example, for preparing azides from primary amines in a click reaction.[4] Diazo reagents can be prepared starting from dimethyl 2-oxopropylphosphonate using fluorosulfonyl azide. If diazabicycloundecene is added as a base, the Ohira-Bestmann reagent is obtained. With magnesium oxide, the Seyferth-Gilbert reagent dimethyldiazomethylphosphonate is obtained.[5]
These reagents are used to convert aldehydes into alkynes. With the two precursors, such a reaction is also possible as a one-pot synthesis. In this case, an aldehyde is added directly and potassium carbonate is used as the base.[6]
See also
References
- ^ Li, Heyin; Wang, Yi (May 2025). "Progress in Radical Fluorosulfonyl Reagents". Synthesis. 57 (10): 1690–1706. doi:10.1055/s-0043-1775417. ISSN 0039-7881. Retrieved 9 August 2025.
- ^ Ruff, John K. (1 April 1965). "Sulfur Oxyfluoride Derivatives. II". Inorganic Chemistry. 4 (4): 567–570. doi:10.1021/ic50026a027. ISSN 0020-1669. Retrieved 9 August 2025.
- ^ Chivers, Tristram; Laitinen, Risto S. (12 October 2021). Chalcogen-nitrogen Chemistry: From Fundamentals To Applications In Biological, Physical And Materials Sciences (Updated ed.). World Scientific. p. 139. ISBN 978-981-12-4135-2. Retrieved 9 August 2025.
- ^ Meng, Genyi; Guo, Taijie; Ma, Tiancheng; Zhang, Jiong; Shen, Yucheng; Sharpless, Karl Barry; Dong, Jiajia (October 2019). "Modular click chemistry libraries for functional screens using a diazotizing reagent". Nature. 574 (7776): 86–89. Bibcode:2019Natur.574...86M. doi:10.1038/s41586-019-1589-1. ISSN 1476-4687. PMID 31578481. Retrieved 9 August 2025.
- ^ Liu, Shuo; Liang, Yufei; Xu, Long; Dong, Jiajia (2024). "Base-Regulated Synthesis of the Bestmann-Ohira or Seyferth-Gilbert Reagent Utilizing FSO2N3". European Journal of Organic Chemistry. 27 (17): e202301282. doi:10.1002/ejoc.202301282. ISSN 1099-0690. Retrieved 9 August 2025.
- ^ Liu, Shuo; Liang, Yufei; Xu, Long; Dong, Jiajia (2024). "Base-Regulated Synthesis of the Bestmann-Ohira or Seyferth-Gilbert Reagent Utilizing FSO2N3". European Journal of Organic Chemistry. 27 (17): e202301282. doi:10.1002/ejoc.202301282. ISSN 1099-0690. Retrieved 9 August 2025.