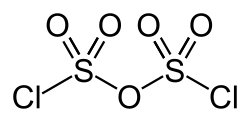
Disulfuryl chloride
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| UN number | 1817 (PYROSULFURYL CHLORIDE) |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cl2O5S2 | |
| Molar mass | 215.02 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.84 g/cm3 |
| Melting point | –37 °C |
| Boiling point | 152.5 °C |
| reacts with water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Disulfuryl chloride is an inorganic compound of sulfur, chlorine, and oxygen with the chemical formula S2O5Cl2.[1] This is the anhydride of chlorosulfuric acid.
Synthesis
Careful heating of sulfur trioxide and carbon tetrachloride:[2]
- 2SO3 + CCl4 → S2O5Cl2 + COCl2
There are also other known methods that do not produce phosgene as a by-product, for example mixing sulfur trioxide and sulfuryl chloride:
- SO3 + SO2Cl2 → S2O5Cl2
Physical properties
The compound appears as a dense, very refractive, colorless liquid with a pungent odor, insoluble in cold water, and prone to hydrolysis.[3][4] Its tendency to smoke in air is low when the compound is pure, while the smoke increases with the presence of chlorosulfuric acid impurities, which are more prone to hydrolysis.
Chemical properties
It slowly drools on contact with water:[5]
- S2O5Cl2 + 3H2O → 2H2SO4 + 2HCl↑
Prolonged boiling or heating to 250 °C results in dissociation into sulfur trioxide, sulfur dioxide, and chlorine.[2]
Uses
The compound is used in organic synthesis and as a chlorinating agent.[5]
See also
- Disulfuryl fluoride
- Disulfuryl chloride fluoride
- Peroxydisulfuryl difluoride
- Trisulfuryl chloride
References
- ^ Bünzli-Trepp, Ursula (1 January 2007). Systematic Nomenclature of Organic, Organometallic and Coordination Chemistry: Chemical-Abstracts Guidelines with IUPAC Recommendations and Many Trivial Names. EPFL Press. p. 290. ISBN 978-1-4200-4615-1. Retrieved 17 August 2025.
- ^ a b Brauer, Georg (1975). Handbuch der Präparativen Anorganischen Chemie (in German) (3., umgearb. Aufl ed.). Stuttgart: Enke. p. 389. ISBN 3-432-02328-6. Retrieved 17 August 2025.
- ^ Lewis, Robert A. (31 May 2016). Hawley's Condensed Chemical Dictionary. John Wiley & Sons. p. 1156. ISBN 978-1-118-13515-0. Retrieved 17 August 2025.
- ^ Craig, Bruce D.; Anderson, David S. (31 December 1994). Handbook of Corrosion Data. ASM International. p. 328. ISBN 978-0-87170-518-1. Retrieved 17 August 2025.
- ^ a b Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 2896. ISBN 978-0-412-30120-9. Retrieved 17 August 2025.