Noribogaine
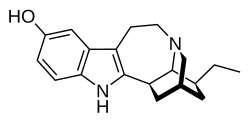 | |
 | |
| Clinical data | |
|---|---|
| Other names | 12-Hydroxyibogamine; Ibogamin-12-ol; O-Desmethylibogaine; O-Demethylibogaine; O-Noribogaine; (–)-Noribogaine |
| Routes of administration | Oral[1][2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 24–50 hours[3][1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H24N2O |
| Molar mass | 296.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Noribogaine, also known as O-desmethylibogaine or 12-hydroxyibogamine, is the principal psychoactive metabolite of the oneirogen ibogaine. It is thought to be involved in the antiaddictive effects of ibogaine-containing plant extracts, such as Tabernanthe iboga.[4][5][6][7]
Use and effects
Noribogaine is the major active metabolite of the oneirogen ibogaine and is thought to be primarily though not exclusively responsible for its effects.[8][9] In contrast to ibogaine, noribogaine has been limitedly evaluated in humans.[8] It was noted in 2007 that administration of noribogaine to humans had not yet been reported.[8] In 2015 and 2016 however, two clinical studies of noribogaine were published.[1][2] It was tested at relatively low doses of 3 to 180 mg in these studies.[1][2] At these doses, no hallucinations, dream-like states, or other hallucinogenic effects were reported.[1][2] Similarly, it produced no μ-opioid receptor agonistic pharmacodynamic effects, such as pupil constriction or analgesia.[1] At higher doses, in the area of 400 to 1,000 mg or more, ibogaine has been reported to produce hallucinogenic effects.[8][10][11]
Adverse effects
Side effects of noribogaine include visual impairment (specifically increased light perception sensitivity), headache, nausea, vomiting, and QT prolongation.[1][2]
Pharmacology
Pharmacodynamics
| Target | Affinity (Ki, nM) | Species |
|---|---|---|
| 5-HT1A | >100,000 (Ki) IA (EC50) |
Rat Human |
| 5-HT1B | >100,000 (Ki) IA (EC50) |
Calf Human |
| 5-HT1D | >100,000 (Ki) IA (EC50) |
Calf Human |
| 5-HT1E | ND (Ki) IA (EC50) |
ND Human |
| 5-HT1F | ND (Ki) IA (EC50) |
ND Human |
| 5-HT2A | >100,000 (Ki) IA (EC50) |
Rat Human |
| 5-HT2B | ND (Ki) IA (EC50) |
ND Human |
| 5-HT2C | >100,000 (Ki) IA (EC50) |
Calf Human |
| 5-HT3 | >100,000 (Ki) ND (EC50) |
Mouse/rat ND |
| 5-HT4 | ND (Ki) IA (EC50) |
ND Human |
| 5-HT5A | ND (Ki) IA (EC50) |
ND Human |
| 5-HT6 | ND (Ki) IA (EC50) |
ND Human |
| 5-HT7 | ND | ND |
| α1A–α1D | ND | ND |
| α2A–α2C | ND | ND |
| β1–β3 | ND | ND |
| D1, D2 | >10,000 | Calf |
| D3 | >100,000 | Calf |
| D4, D5 | ND | ND |
| H1–H4 | ND | ND |
| M1 | 15,000 | Calf |
| M2 | 36,000 | Calf |
| M3–M5 | ND | ND |
| nACh | ND (Ki) 6,820 (IC50) |
ND Human |
| I1, I2 | ND | ND |
| σ1 | 11,000–15,006 | Calf/guinea pig |
| σ2 | 5,226–19,000 | Calf/rat |
| MOR | 1,520 (Ki) 7,420–16,050 (EC50) 3–36% (Emax) |
Human Human Human |
| DOR | 5,200–24,720 (Ki) IA (EC50) |
Calf Human |
| KOR | 720 (Ki) 110–8,749 (EC50) 13–85% (Emax) |
Human Human Human |
| NOP | >100,000 | Bovine |
| TAAR1 | ND | ND |
| PCP | 5,480–38,200 | Rat/bovine/human |
| SERT | 41 (Ki) 280–326 (IC50) 840 or IA (EC50) ~30% or IA (Emax) |
Human Human Human Human |
| NET | ND (Ki) 39,000 (IC50) ND (EC50) |
ND Bovine ND |
| DAT | 2,050 (Ki) 6,760 (IC50) ND (EC50) |
Human Human ND |
| VMAT2 | 570–29,500 (IC50) | Human |
| OCT2 | 6,180 (IC50) | Human |
| VGSC | 17,000 (Ki) | Bovine |
| VGCC | ND (IC50) | ND |
| hERG | 1,960 (Ki) 2,860 (IC50) |
Human Human |
| Notes: The smaller the value, the more avidly the drug binds to the site. All proteins are human unless otherwise specified. Refs: [12][13][3][9][14][15][16][17][18][19] [20][21][22][23][24][25][26] | ||
Noribogaine has been determined to act as a biased agonist of the κ-opioid receptor (KOR).[27] It activates the G protein (GDP-GTP exchange) signaling pathway with 75% the efficacy of dynorphin A (EC50 = 9 μM), but it is only 12% as efficacious at activating the β-arrestin pathway.[27] With an IC50 value of 1 μM, it can be regarded as an antagonist of the latter pathway.[27]
The β-arrestin signaling pathway is hypothesized to be responsible for the anxiogenic, dysphoric, or anhedonic effects of KOR activation.[28] Attenuation of the β-arrestin pathway by noribogaine may be the reason for the absence of these aversive effects,[27] while retaining analgesic and antiaddictive properties. This biased KOR activity makes it stand out from the other iboga alkaloids like ibogaine and the derivative 18-methoxycoronaridine (18-MC).[27]
Noribogaine is a potent serotonin reuptake inhibitor,[29] but does not affect the reuptake of dopamine.[30] Unlike ibogaine, noribogaine does not bind to the sigma-2 receptor.[31][32] Similarly to ibogaine, noribogaine acts as a weak NMDA receptor antagonist and binds to opioid receptors.[33] It has greater affinity for each of the opioid receptors than does ibogaine.[34] Noribogaine has been reported to be a low-efficacy serotonin releasing agent, although findings are conflicting and other studies have found that it is inactive as a serotonin releasing agent.[25][24]
Noribogaine is a hERG inhibitor and appears at least as potent as ibogaine.[35] The inhibition of the hERG potassium channel delays the repolarization of cardiac action potentials, resulting in QT interval prolongation and, subsequently, in arrhythmias and sudden cardiac arrest.[36]
Noribogaine has been reported to be a potent psychoplastogen similarly to ibogaine.[18][17][37][25]
Ibogaine and the structurally related hallucinogen harmaline are tremorigenic, whereas noribogaine is not or is much less so.[17][16][38][39]
Pharmacokinetics
Noribogaine is highly lipophilic and shows high brain penetration in rodents.[15][3]
The elimination half-life of noribogaine is 24 to 50 hours.[3][1][2]
History
Noribogaine was first described in the scientific literature by at least 1958.[40][39] It was first identified and described as a metabolite of ibogaine by 1995.[41][42][34][43] The first evaluation of noribogaine in humans was published in 2015.[1][2]
See also
- Iboga alkaloid
- Ibogamine
- Voacangine
- Tabernanthine
- Coronaridine
- Oxa-noribogaine
- RB-64
- 6'-Guanidinonaltrindole
- Herkinorin
- TRV130
- Nalfurafine
- GM-3009
References
- ^ a b c d e f g h i Glue P, Lockhart M, Lam F, Hung N, Hung CT, Friedhoff L (February 2015). "Ascending-dose study of noribogaine in healthy volunteers: pharmacokinetics, pharmacodynamics, safety, and tolerability". Journal of Clinical Pharmacology. 55 (2): 189–194. doi:10.1002/jcph.404. PMID 25279818.
- ^ a b c d e f g h Glue P, Cape G, Tunnicliff D, Lockhart M, Lam F, Hung N, et al. (November 2016). "Ascending Single-Dose, Double-Blind, Placebo-Controlled Safety Study of Noribogaine in Opioid-Dependent Patients". Clinical Pharmacology in Drug Development. 5 (6): 460–468. doi:10.1002/cpdd.254. PMID 27870477.
Visual changes involving change in light perception were reported shortly after dosing, mainly by subjects dosed with 120–180 mg. These changes only occurred during the drug absorption phase, being first reported 1 hour after dosing, and had disappeared by 2.5–3 hours. No hallucinations or dream-like states were reported. In contrast higher ibogaine doses produced symptoms including light sensitivity and closed-eyed dream-like states for 4–8 hours.15
- ^ a b c d Wasko MJ, Witt-Enderby PA, Surratt CK (October 2018). "DARK Classics in Chemical Neuroscience: Ibogaine". ACS Chemical Neuroscience. 9 (10): 2475–2483. doi:10.1021/acschemneuro.8b00294. PMID 30216039.
Unlike LSD, mescaline, and psilocybin, the hallucinogenic properties of ibogaine cannot be ascribed to 5-HT2A receptor activation.
- ^ Mash DC, Ameer B, Prou D, Howes JF, Maillet EL (Jul 2016). "Oral noribogaine shows high brain uptake and anti-withdrawal effects not associated with place preference in rodents". Journal of Psychopharmacology. 30 (7). Oxford, England: 688–697. doi:10.1177/0269881116641331. PMID 27044509. S2CID 40776971.
- ^ Glick SD, Maisonneuve IS (May 1998). "Mechanisms of antiaddictive actions of ibogaine". Annals of the New York Academy of Sciences. 844 (1): 214–226. Bibcode:1998NYASA.844..214G. doi:10.1111/j.1749-6632.1998.tb08237.x. PMID 9668680. S2CID 11416176.
- ^ Baumann MH, Pablo J, Ali SF, Rothman RB, Mash DC (2001). "Comparative neuropharmacology of ibogaine and its O-desmethyl metabolite, noribogaine". The Alkaloids. Chemistry and Biology. 56: 79–113. doi:10.1016/S0099-9598(01)56009-5. PMID 11705118.
- ^ Kubiliene A, Marksiene R, Kazlauskas S, Sadauskiene I, Razukas A, Ivanov L (2008). "Acute toxicity of ibogaine and noribogaine". Medicina. 44 (12). Kaunas, Lithuania: 984–988. doi:10.3390/medicina44120123. PMID 19142057.
- ^ a b c d Alper, K. R., & Lotsof, H. S. (2007). The use of ibogaine in the treatment of addictions. Psychedelic Medicine: New Evidence for Hallucinogenic Substances as Treatments, 2, 43–66. https://web.archive.org/web/20220828090846/https://s3.ca-central-1.amazonaws.com/ibosafe-pdf-resources/Ibogaine/The+use+of+ibogaine+in+the+treatment+of+addictions.pdf
- ^ a b Glick SD, Maisonneuve IM, Szumlinski KK (2001). "Mechanisms of action of ibogaine: Relevance to putative therapeutic effects and development of a safer iboga alkaloid congener" (PDF). The Alkaloids. Chemistry and Biology. 56: 39–53. doi:10.1016/S0099-9598(01)56006-X. ISBN 978-0-12-469556-6. ISSN 1099-4831. OCLC 119074996. PMID 11705115. Archived from the original (PDF) on 5 April 2014.
Indeed, an active metabolite of ibogaine, noribogaine, has already been well characterized both in vivo (e.g., 2,3) and in vitro (e.g., 35,36). Although some investigators (37) consider noribogaine to be the major determinant of ibogaine's pharmacology in vivo, studies in this laboratory (20) indicated that the elimination of noribogaine was also too fast for it to be responsible for all of ibogaine's prolonged effects. [...] The short-half lives of ibogaine and 18-MC strongly suggest that the pharmacological actions of both alkaloids are attributable to one or more active metabolites; although noribogaine has been proposed (2,37) as the mediator of ibogaine's prolonged action, it would appear that noribogaine alone cannot account for ibogaine's effects since brain levels of noribogaine also decline rapidly after ibogaine administration to rats (20).
- ^ Shulgin A, Shulgin A (September 1997). TiHKAL: The Continuation. Berkeley, California: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252.
- ^ Shulgin AT (2003). "Basic Pharmacology and Effects". In Laing RR (ed.). Hallucinogens: A Forensic Drug Handbook. Forensic Drug Handbook Series. Elsevier Science. pp. 67–137. ISBN 978-0-12-433951-4.
Ibogaine is an active hallucinogen in the 400 milligram area and has been clinically studied for the treatment of heroin addiction. In this latter role, the dosages employed may range as high as 1500mg. A primary human metabolism is via O-demethylation to give the free phenol 12-hydroxyibogamine. This metabolite, misnamed nor-ibogaine in the literature, appears to be pharmacologically active in its own right.
- ^ "Kᵢ Database". PDSP. 31 July 2025. Retrieved 31 July 2025.
- ^ Liu T. "BindingDB BDBM50067814 17-ethyl-(1R,17S)-3,13-diazapentacyclo[13.3.1.02,10.04,9.013,18]nonadeca-2(10),4(9),5,7-tetraen-7-ol (noribogaine)::CHEMBL343956". BindingDB. Retrieved 31 July 2025.
- ^ Glick SD, Maisonneuve IM, Szumlinski KK (September 2000). "18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action". Annals of the New York Academy of Sciences. 914: 369–386. doi:10.1111/j.1749-6632.2000.tb05211.x. PMID 11085336.
- ^ a b Litjens RP, Brunt TM (2016). "How toxic is ibogaine?". Clinical Toxicology. 54 (4). Philadelphia, Pa.: 297–302. doi:10.3109/15563650.2016.1138226. PMID 26807959.
- ^ a b Popik P, Layer RT, Skolnick P (June 1995). "100 years of ibogaine: neurochemical and pharmacological actions of a putative anti-addictive drug". Pharmacological Reviews. 47 (2): 235–253. doi:10.1016/S0031-6997(25)06842-5. PMID 7568327.
Like the structurally relate harmaline, ibogaine produces tremors. In mice, ibogaine is tremorigenic, both when given intracerebrally (ED50 127 nmol/g brain, pg/g with a latency to tremor of about 1 min) (Singbarth et al., 1973) and systemically (ED50 12 mg/kg subcutaneous) (Zetler et al., 1972). Zetler et al. (1972) also established the tremorigenic structure-activity relationship of several ibogaine-like compounds, with the descending order of potency: tabernanthine > ibogaline > ibogaine > iboxygaine > noribogaine. Recently, Glick et al. (1994) found that, whereas ibogaine and tabernanthine produced tremors, ibogamine and coronaridine were devoid of such an effect.
- ^ a b c Iyer RN, Favela D, Zhang G, Olson DE (March 2021). "The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs". Natural Product Reports. 38 (2): 307–329. doi:10.1039/d0np00033g. PMC 7882011. PMID 32794540.
- ^ a b Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. PMC 7874389. PMID 33299186.
- ^ Staley JK, Ouyang Q, Pablo J, Hearn WL, Flynn DD, Rothman RB, et al. (September 1996). "Pharmacological screen for activities of 12-hydroxyibogamine: a primary metabolite of the indole alkaloid ibogaine". Psychopharmacology. 127 (1): 10–18. doi:10.1007/BF02805969. PMID 8880938.
- ^ Antonio T, Childers SR, Rothman RB, Dersch CM, King C, Kuehne M, et al. (2013). "Effect of Iboga alkaloids on µ-opioid receptor-coupled G protein activation". Plos One. 8 (10): e77262. Bibcode:2013PLoSO...877262A. doi:10.1371/journal.pone.0077262. PMC 3818563. PMID 24204784.
- ^ Maillet EL, Milon N, Heghinian MD, Fishback J, Schürer SC, Garamszegi N, et al. (December 2015). "Noribogaine is a G-protein biased κ-opioid receptor agonist". Neuropharmacology. 99: 675–688. doi:10.1016/j.neuropharm.2015.08.032. PMID 26302653.
- ^ Mash DC, Staley JK, Pablo JP, Holohean AM, Hackman JC, Davidoff RA (June 1995). "Properties of ibogaine and its principal metabolite (12-hydroxyibogamine) at the MK-801 binding site of the NMDA receptor complex". Neuroscience Letters. 192 (1): 53–56. doi:10.1016/0304-3940(95)11608-y. PMID 7675310.
- ^ Wells GB, Lopez MC, Tanaka JC (April 1999). "The effects of ibogaine on dopamine and serotonin transport in rat brain synaptosomes". Brain Research Bulletin. 48 (6): 641–647. doi:10.1016/s0361-9230(99)00053-2. PMID 10386845.
- ^ a b Hwu C, Havel V, Westergaard X, Mendieta AM, Serrano IC, Hwu J, et al. (10 March 2025), "Deciphering Ibogaine's Matrix Pharmacology: Multiple Transporter Modulation at Serotonin Synapses", bioRxiv, doi:10.1101/2025.03.04.641351
- ^ a b c Iyer RN, Favela D, Domokos A, Zhang G, Avanes AA, Carter SJ, et al. (March 2025). "Efficient and modular synthesis of ibogaine and related alkaloids". Nature Chemistry. 17 (3): 412–420. doi:10.1038/s41557-024-01714-7. PMID 39915657.
- ^ Alper K, Bai R, Liu N, Fowler SJ, Huang XP, Priori SG, et al. (January 2016). "hERG Blockade by Iboga Alkaloids". Cardiovascular Toxicology. 16 (1): 14–22. doi:10.1007/s12012-015-9311-5. PMID 25636206.
- ^ a b c d e Maillet EL, Milon N, Heghinian MD, Fishback J, Schürer SC, Garamszegi N, et al. (Dec 2015). "Noribogaine is a G-protein biased κ-opioid receptor agonist". Neuropharmacology. 99: 675–688. doi:10.1016/j.neuropharm.2015.08.032. PMID 26302653.
- ^ Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, et al. (Sep 2015). "Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons". The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 35 (37): 12917–12931. doi:10.1523/JNEUROSCI.2444-15.2015. PMC 4571610. PMID 26377476.
- ^ Houck MM (26 January 2015). Forensic Chemistry. Elsevier Science. pp. 164–. ISBN 978-0-12-800624-5.
- ^ Baumann MH, Rothman RB, Pablo JP, Mash DC (May 2001). "In vivo neurobiological effects of ibogaine and its O-desmethyl metabolite, 12-hydroxyibogamine (noribogaine), in rats". The Journal of Pharmacology and Experimental Therapeutics. 297 (2): 531–539. PMID 11303040.
- ^ Gahlinger P (30 December 2003). Illegal Drugs. Penguin Publishing Group. pp. 304–. ISBN 978-1-4406-5024-6.
- ^ Alper KR, Glick SD (2001). Ibogaine: Proceedings from the First International Conference. Gulf Professional Publishing. pp. 107–. ISBN 978-0-12-053206-3.
- ^ Barceloux DG (20 March 2012). Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. John Wiley & Sons. pp. 869–. ISBN 978-0-471-72760-6.
- ^ a b Pearl SM, Herrick-Davis K, Teitler M, Glick SD (March 1995). "Radioligand-binding study of noribogaine, a likely metabolite of ibogaine". Brain Research. 675 (1–2): 342–344. doi:10.1016/0006-8993(95)00123-8. PMID 7796150. S2CID 28001919.
- ^ Alper K, Bai R, Liu N, Fowler SJ, Huang XP, Priori SG, et al. (Jan 2016). "hERG Blockade by Iboga Alkaloids". Cardiovascular Toxicology. 16 (1): 14–22. doi:10.1007/s12012-015-9311-5. PMID 25636206. S2CID 16071274.
- ^ Litjens RP, Brunt TM (2016). "How toxic is ibogaine?". Clinical Toxicology. 54 (4). Philadelphia, Pa.: 297–302. doi:10.3109/15563650.2016.1138226. PMID 26807959. S2CID 7026570.
- ^ Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. (June 2018). "Psychedelics Promote Structural and Functional Neural Plasticity". Cell Reports. 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. PMC 6082376. PMID 29898390.
- ^ Baumann MH, Pablo JP, Ali SF, Rothman RB, Mash DC (September 2000). "Noribogaine (12-hydroxyibogamine): a biologically active metabolite of the antiaddictive drug ibogaine". Annals of the New York Academy of Sciences. 914: 354–368. doi:10.1111/j.1749-6632.2000.tb05210.x. PMID 11085335.
- ^ a b Zetler G, Singbartl G, Schlosser L (1972). "Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids". Pharmacology. 7 (4): 237–248. doi:10.1159/000136294. PMID 5077309.
- ^ Bartlett MF, Dickel DF, Taylor WI (1958). "The Alkaloids of Tabernanthe iboga. Part IV. 1 The Structures of Ibogamine, Ibogaine, Tabernanthine and Voacangine". Journal of the American Chemical Society. 80 (1): 126–136. doi:10.1021/ja01534a036. ISSN 0002-7863. Retrieved 1 August 2025.
- ^ Mash DC, Staley JK, Baumann MH, Rothman RB, Hearn WL (1995). "Identification of a primary metabolite of ibogaine that targets serotonin transporters and elevates serotonin". Life Sciences. 57 (3): PL45 – PL50. doi:10.1016/0024-3205(95)00273-9. PMID 7596224.
- ^ Hearn WL, Pablo J, Hime GW, Mash DC (October 1995). "Identification and quantitation of ibogaine and an o-demethylated metabolite in brain and biological fluids using gas chromatography-mass spectrometry". Journal of Analytical Toxicology. 19 (6): 427–434. doi:10.1093/jat/19.6.427. PMID 8926737.
- ^ Rezvani AH, Mash DC, Hearn WL, Lee YW, Overstreet DH (1995). "Noribogaine, a primary Ibogaine metabolite, reduces alcohol intake in P and fawn-hooded rats". Alcohol Clin. Exp. Res. 19: 15A.