Dordaviprone
 | |
| Clinical data | |
|---|---|
| Trade names | Modeyso |
| Other names | ONC201, ONC-201 |
| AHFS/Drugs.com | Modeyso |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Protease activator |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
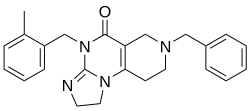
| Formula | C24H26N4O |
| Molar mass | 386.499 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dordaviprone, sold under the brand name Modeyso is an anti-cancer medication used for the treatment of diffuse midline glioma (a type of brain tumor).[1][2] Dordaviprone is a protease activator of the mitochondrial caseinolytic protease P.[1] It is dopamine receptor D2 antagonist and an allosteric activator of the mitochondrial caseinolytic protease P.[3]
Dordaviprone was approved for medical use in the United States in August 2025.[2] It is the first approval of a systemic therapy for H3 K27M-mutant diffuse midline glioma by the US Food and Drug Administration.[2]
Medical uses
Dordaviprone is indicated for the treatment of people with diffuse midline glioma harboring an H3 K27M mutation with progressive disease following prior therapy.[1][2]
History
Efficacy was evaluated in an integrated efficacy population of 50 participants with recurrent H3 K27M-mutant diffuse midline glioma enrolled across five open-label, non-randomized clinical trials conducted in the US (ONC006 [NCT02525692], ONC013 [NCT03295396], ONC014 [NCT03416530], ONC016 [NCT05392374], and ONC018 [NCT03134131]).[2] The efficacy population comprised participants who received single-agent dordaviprone for diffuse midline glioma harboring an H3 K27M mutation and had progressive and measurable disease per Response Assessment in Neuro-Oncology-High Grade Glioma (RANO-HGG) criteria.[2] Participants were also at least 90 days post radiation therapy, had an adequate washout period from prior anticancer therapies, a Karnofsky Performance Status/Lansky Performance Status (KPS/LPS) score ≥ 60, and stable or decreasing corticosteroid use.[2] Participants with diffuse intrinsic pontine glioma, primary spinal tumors, atypical histologies, or cerebrospinal fluid dissemination were excluded.[2]
The US Food and Drug Administration granted the application for dordaviprone priority review, orphan drug, rare pediatric disease, and fast track designations.[2]
Society and culture
Legal status
Dordaviprone was approved for medical use in the United States in August 2025.[2][4]
Names
Dordaviprone is the international nonproprietary name.[5]
Dordaviprone is sold under the brand name Modeyso.[2][4]
References
- ^ a b c d https://pp.jazzpharma.com/pi/modeyso.en.USPI.pdf
- ^ a b c d e f g h i j k "FDA grants accelerated approval to dordaviprone for diffuse midline glioma". U.S. Food and Drug Administration (FDA). 6 August 2025. Retrieved 7 August 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Prabhu VV, Morrow S, Rahman Kawakibi A, Zhou L, Ralff M, Ray J, et al. (December 2020). "ONC201 and imipridones: Anti-cancer compounds with clinical efficacy". Neoplasia. 22 (12). New York, N.Y.: 725–744. doi:10.1016/j.neo.2020.09.005. PMC 7588802. PMID 33142238.
- ^ a b "Jazz Pharmaceuticals Announces U.S. FDA Approval of Modeyso (dordaviprone) as the First and Only Treatment for Recurrent H3 K27M-mutant Diffuse Midline Glioma" (Press release). Jazz Pharmaceuticals. 6 August 2025. Retrieved 10 August 2025 – via PR Newswire.
- ^ World Health Organization (2023). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 89". WHO Drug Information. 37 (1). hdl:10665/366661.
External links
- Clinical trial number NCT02525692 for "Oral ONC201 in Adult Recurrent Glioblastoma" at ClinicalTrials.gov
- Clinical trial number NCT03295396 for "ONC201 in Adults With Recurrent H3 K27M-mutant Glioma" at ClinicalTrials.gov
- Clinical trial number NCT03416530 for "ONC201 in Pediatric H3 K27M Gliomas" at ClinicalTrials.gov
- Clinical trial number NCT05392374 for "Expanded Access Use of ONC201 in a Patient With Diffuse Intrinsic Pontine Gliomas" at ClinicalTrials.gov
- Clinical trial number NCT03134131 for "Expanded Access to ONC201 for Patients With H3 K27M-mutant and/or Midline High Grade Gliomas" at ClinicalTrials.gov