Plitidepsin
 | |
| Names | |
|---|---|
| Systematic IUPAC name
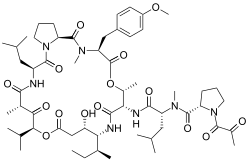
(2S)-N-[(1R)-1-({(3S,6R,7S,10R,11S,15S,17S,20S,25aS)-10-[(2S)-Butan-2-yl]-11-hydroxy-3-[(4-methoxyphenyl)methyl]-2,6,17-trimethyl-20-(2-methylpropyl)-1,4,8,13,16,18,21-heptaoxo-15-(propan-2-yl)docosahydro-15H-pyrrolo[2,1-d] [10,19,1,4,7,14]dioxatetraazacyclotricosin-7-yl}carbamoyl)-3-methylbutyl]-N-methyl-1-(2-oxopropanoyl)pyrrolidine-2-carboxamide | |
| Other names
Aplidine; Aplidin, dehydrodidemnin B; Aplidin; N-[1-(1,2-Dioxopropyl)-L-prolyl]didemnin A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C57H87N7O15 | |
| Molar mass | 1110.357 g·mol−1 |
| Pharmacology | |
| L01XX57 (WHO) | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Plitidepsin, also known as dehydrodidemnin B and sold under the brand name Aplidin, is a chemical compound extracted from the ascidian Aplidium albicans.[3]
Medical uses
In Australia, plitidepsin, in combination with dexamethasone, is indicated for the treatment of people with relapsed and refractory multiple myeloma.[1][2][4]
Pharmacological activity
Plitidepsin exhibits antitumor, antiviral and immunosuppressive activities. It shows promise in shrinking tumors in pancreatic, stomach, bladder, and prostate cancers.[5][6]
Plitidepsin inhibits the human protein eEF1A which has potential interactions with multiple coronavirus proteins. Plitidepsin possesses antiviral activity against SARS-CoV-2 in vitro and in an in vivo mouse model.[7]
Society and culture
Legal status
In July 2003, plitidepsin was granted orphan drug status by the European Medicines Agency (EMA) for treating acute lymphoblastic leukemia.[8] In December 2017, the EMA's Committee for Medicinal Products for Human Use (CHMP) adopted a negative opinion, recommending the refusal of the marketing authorization for the treatment of multiple myeloma.[9] After a re-examination of the opinion, the refusal of the marketing authorization was confirmed in March 2018.[9] The CHMP is of the opinion that the benefits of Aplidin do not outweigh its risks.[9] In October 2020, the General Court upheld PharmaMar's appeal and annulled the decision refusing marketing authorization for Aplidin, and the European Commission then returned the application for Aplidin to the EMA.[9][10] In July 2025, PharmaMar withdrew its application for a marketing authorization of Aplidin for the treatment of multiple myeloma.[9]
Plitidepsin was approved for medical used in Australia in December 2018.[2]
References
- ^ a b c https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2018-PI-02713-1
- ^ a b c d "AusPAR: Plitidepsin". Therapeutic Goods Administration (TGA). 8 July 2019.
- ^ Newman DJ, Cragg GM (August 2004). "Marine natural products and related compounds in clinical and advanced preclinical trials". Journal of Natural Products. 67 (8): 1216–38. doi:10.1021/np040031y. PMID 15332835.
- ^ "Aplidin (Specialised Therapeutics Pharma Pty Ltd)". Therapeutic Goods Administration (TGA). 24 July 2025. Retrieved 27 July 2025.
- ^ Garrison T (2002). Oceanography: An Invitation to Marine Science (4th ed.). United States: Brooks/Cole. p. 98.
- ^ Adrio J, Cuevas C, Manzanares I, Joullié MM (July 2007). "Total synthesis and biological evaluation of tamandarin B analogues". The Journal of Organic Chemistry. 72 (14): 5129–38. doi:10.1021/jo070412r. PMID 17555353.
- ^ White KM, Rosales R, Yildiz S, Kehrer T, Miorin L, Moreno E, et al. (January 2021). "Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A". Science. 371 (6532): 926–931. Bibcode:2021Sci...371..926W. doi:10.1126/science.abf4058. PMC 7963220. PMID 33495306.
- ^ "Public Summary of Positive Opinion for Orphan Designation of Aplidine for the Treatment of Acute Lymphoblastic Leukaemia" (PDF). European Medicines Agency (EMA). 1 September 2011.
- ^ a b c d e "Aplidin EPAR". European Medicines Agency (EMA).
- ^ "Aplidin". European Medicines Agency. 28 October 2020. Retrieved 11 July 2024.
Further reading
Delgado-Calle J, Kurihara N, Atkinson EG, Nelson J, Miyagawa K, Galmarini CM, et al. (April 2019). "Aplidin (plitidepsin) is a novel anti-myeloma agent with potent anti-resorptive activity mediated by direct effects on osteoclasts". Oncotarget. 10 (28): 2709–2721. doi:10.18632/oncotarget.26831. PMC 6505631. PMID 31105871.