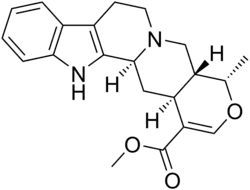
Ajmalicine Routes of Oral ATC code Legal status
In general: ℞ (Prescription only)
(19α)-16,17-didehydro- 19-methyloxayohimban- 16-carboxylic acid methyl ester
CAS Number PubChem CID ChemSpider UNII ChEBI ChEMBL CompTox Dashboard (EPA ) ECHA InfoCard 100.006.900 Formula C 21 H 24 N 2 O 3 Molar mass −1 3D model (JSmol ) Melting point 262.5 to 263 °C (504.5 to 505.4 °F)
O=C(OC)\C4=C\OC(C5CN3CCc1c([nH]c2ccccc12)C3CC45)C
InChI=1S/C21H24N2O3/c1-12-16-10-23-8-7-14-13-5-3-4-6-18(13)22-20(14)19(23)9-15(16)17(11-26-12)21(24)25-2/h3-6,11-12,15-16,19,22H,7-10H2,1-2H3/t12-,15-,16+,19-/m0/s1
Y Key:GRTOGORTSDXSFK-XJTZBENFSA-N
Y N Y (verify)
Ajmalicine , also known as δ-yohimbine or raubasine , is an antihypertensive drug used in the treatment of high blood pressure .[ 1] Card-Lamuran , Circolene , Cristanyl , Duxil , Duxor , Hydroxysarpon , Iskedyl , Isosarpan , Isquebral , Lamuran , Melanex , Raunatin , Saltucin Co , Salvalion , and Sarpan .[ 1] alkaloid found naturally in various plants such as Rauvolfia Catharanthus roseus Mitragyna speciosa [ 1] [ 2] [ 3]
Ajmalicine is structurally related to yohimbine , rauwolscine , and other yohimban derivatives .[ 4] corynanthine , it acts as a α1 -adrenergic receptor antagonist with preferential actions over α2 -adrenergic receptors , underlying its hypotensive rather than hypertensive effects.[ 1] [ 5]
Additionally, it is a very strong inhibitor of the CYP2D6 liver enzyme, which is responsible for the breakdown of many drugs. Its binding affinity at this receptor is 3.30 nM.[ 6]
Biosynthesis
Two moieties are involved in the biosynthesis of ajmalicine, the terpenoid moiety and the indole moiety.[ 7]
Biosynthetic pathway of ajmalicine. Reconstruction of figure 1 in Chang, K. (2014).[ 7]
See also
References
^ a b c d Wink M, Roberts MW (1998). "Compartmentation of alkaloid synthesis, transport. and storage" . Alkaloids: biochemistry, ecology, and medicinal applications . New York: Plenum Press. ISBN 0-306-45465-3 . ^ Kurz WG, Chatson KB, Constabel F, Kutney JP, Choi LS, Kolodziejczyk P, et al. (May 1981). "Alkaloid Production in Catharanthus roseus cell cultures VIII". Planta Medica . 42 (1): 22– 31. doi :10.1055/s-2007-971541 . PMID 17401876 . S2CID 28177495 . ^ León F, Habib E, Adkins JE, Furr EB, McCurdy CR, Cutler SJ (July 2009). "Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A" . Natural Product Communications . 4 (7): 907– 910. doi :10.1177/1934578X0900400705 PMC 9255435 PMID 19731590 . S2CID 37709142 . ^ Roberts MF (1998-06-30). Alkaloids: Biochemistry, Ecology, and Medicinal Applications . Springer Science & Business Media. ISBN 978-0-306-45465-3 . ^ Roquebert J, Demichel P (October 1984). "Inhibition of the alpha 1 and alpha 2-adrenoceptor-mediated pressor response in pithed rats by raubasine, tetrahydroalstonine and akuammigine". European Journal of Pharmacology . 106 (1): 203– 205. doi :10.1016/0014-2999(84)90698-8 . PMID 6099269 . ^ Strobl GR, von Kruedener S, Stöckigt J, Guengerich FP, Wolff T (April 1993). "Development of a pharmacophore for inhibition of human liver cytochrome P-450 2D6: molecular modeling and inhibition studies". Journal of Medicinal Chemistry . 36 (9): 1136– 1145. doi :10.1021/jm00061a004 . PMID 8487254 . ^ a b Chang K, Chen M, Zeng L, Lan X, Wang Q, Liao Z (2014). "Abscisic Acid Enhanced Ajmalicine Biosynthesis in Hairy Roots of Rauvolfia verticillata by Upregulating Expression of the MEP Pathway Genes". Russian Journal of Plant Physiology . 61 (1): 136– 141. doi :10.1134/S102144371401004X . ISSN 1021-4437 . S2CID 255013940 .
α1
Agonists Antagonists
Abanoquil Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β
See also: Receptor/signaling modulatorsDopaminergics Serotonergics Monoamine reuptake inhibitors Monoamine releasing agents Monoamine metabolism modulators Monoamine neurotoxins
Tryptamines 4-Hydroxytryptamines esters /ethers 5-Hydroxy- and5-methoxytryptamines
2-Methyl-5-HT 4-HO-5-MeO-T 4-F-5-MeO-DMT 4,5-DHP-DMT 4,5-DHT 4,5-MDO-DMT 4,5-MDO-DiPT 5-BT 5-Ethoxy-DMT 5-HO-DET 5-HO-DiPT 5-HO-NiPT
5-HO-DPT 5-HTP (oxitriptan )5-MeO-2-TMT 5-MeO-34MPEMT
5-MeO-7,N ,N -TMT 5-MeO-DALT 5-MeO-DBT 5-MeO-DET 5-MeO-DiPT 5-MeO-DMT (N ,N ,O -TMS; O -methylbufotenine) 5-MeO-DPT 5-MeO-EiPT 5-MeO-EPT 5-MeO-MALT 5-MeO-MET 5-MeO-MiPT 5-MeO-NET 5-MeO-NiPT 5-MeO-NMT (O ,N -DMS) 5-MeO-PiPT 5-MeO-NBpBrT 5-MeO-T (5-MT; mexamine; O -methylserotonin) 5-MeO-T-NBOMe 5-MT-NB3OMe
5-NOT 5,6-DHT 5,6-MDO-DiPT 5,6-MDO-DMT 5,6-MDO-MiPT 5,6-MeO-MiPT 5,7-DHT Arachidonoyl serotonin ASR-3001 (5-MeO-iPALT) BAB
Benanserin (BAS; SQ-4788) BGC20-761 Bufotenidine (5-HTQ; N ,N ,N -TMS) Bufotenin (5-HO-DMT; N ,N -DMS; mappine) Bufoviridine (5-SO-DMT) CP-132,484 Cqd 280
Cqd 285
Cqdd 280
Donitriptan EMDT (2-Et-5-MeO-DMT) HIOC Indorenate (TR-3369) Isamide (N -CA-5-MT) L-741604 MS-245 N -DEAOP-5-MeO-NETN -DEAOP-5-MeO-NMTN -Feruloylserotonin (moschamine)Norbufotenin (5-HO-NMT; NMS) O -Acetylbufotenine (5-AcO-DMT)O -Pivalylbufotenine (5-(t -BuCO)-DMT)Psilomethoxin (4-HO-5-MeO-DMT) Psilomethoxybin (4-PO-5-MeO-DMT) Serotonin (5-HT) N -Acetyltryptaminesα-Alkyltryptamines
5-Hydroxy- and 5-alkoxy-α-alkyltryptamines: 1-Pr-5-MeO-AMT 5-Allyloxy-AMT 5-Ethoxy-αMT 5-iPrO-αMT
5-MeO-αET 5-MeO-αMT (α,O -DMS; Alpha-O) α-Methyl-5-HTP α-Methylmelatonin α-Methylserotonin (5-HO-αMT; α-Me-5-HT) α,N ,O -TMS (5-MeO-α,N -DMT) α,N ,N ,O -TeMS (5-MeO-α,N ,N -TMT) AL-37350A (4,5-DHP-αMT) BW-723C86 Cyclized tryptamines
Barettin Cyclic 3-OHM Ergolines and lysergamides (e.g., LSD )Harmala alkaloidsβ-carbolines (e.g., 5-methoxyharmalan , 6-MeO-THH , 6-methoxyharmalan , 9-Me-BC , β-carboline (norharman) , fenharmane , harmaline , harmalol , harmane , harmine , LY-266,097 , pinoline , tetrahydroharmine , tryptoline )Iboga alkaloidsibogaine , ibogamine , noribogaine , tabernanthine )Ibogalogs (e.g., catharanthalog , fluorogainalog, ibogainalog , ibogaminalog (DM-506) , LS-22925, noribogainalog , noribogaminalog , PHA-57378 , PNU-22394 , tabernanthalog )Imidazolylindoles (e.g., AGH-107 , AGH-192 , AH-494 )Metralindole Partial ergolines and lysergamides (e.g., NDTDI , RU-27849 , RU-28251 , RU-28306 , FHATHBIN , LY-178210 , Bay R 1531 (LY-197206) , LY-293284 , 10,11-seco-LSD , 10,11-secoergoline (α,N -Pip-T) , CT-5252 )Pertines (e.g., alpertine , milipertine , oxypertine , solypertine )Piperidinylethylindoles (e.g., pip-T , indolylethylfentanyl)Pyrrolidinylethylindoles (e.g., pyr-T , 4-HO-pyr-T , 5-MeO-pyr-T , 4-F-5-MeO-pyr-T )Pyrrolidinylmethylindoles (e.g., MPMI , 4-HO-MPMI (lucigenol) , 5F-MPMI , 5-MeO-MPMI , CP-122288 , CP-135807 , eletriptan )Tetrahydrocarbazolamines (e.g., ciclindole , flucindole , frovatriptan , LY-344864 , ramatroban )Tetrahydropyridinylindoles (e.g., RS134-49 , RU-28253 )Tetrahydropyrroloquinolines (e.g., bufothionine , O -methylnordehydrobufotenineYohimbans (e.g., yohimbine , rauwolscine , spegatrine , corynanthine , , reserpine , deserpidine , rescinnamine ) Isotryptamines Related compounds
2-Azapsilocin
4-Aza-5-MeO-DPT
5-Aza-4-MeO-DiPT
5-HIAA 5-HIAL 5-HITCA
5-MIAL
7-Aza-5-MeO-DiPT
α-Carboline
γ-Carbolines (pyridoindoles) (e.g., γ-carboline , alosetron , gevotroline , latrepirdine , lurosetron , mebhydrolin , tiflucarbine )Amedalin Benzindopyrine
Benzofurans (e.g., 3-APB , 5-MeO-DiBF , BPAP , 3-F-BPAP , dimemebfe , mebfap , oxa-noribogaine )Benzothiophenes (e.g., 3-APBT )Carmoxirole CT-4436
Daledalin Gramine Histamine I-32
IAL IN-399
Indazolethylamines (e.g., AL-34662 , AL-38022A , O -methyl-AL-34662VU6067416 , YM-348 )Indenylethylamines (e.g., C-DMT )Indolizinylethylamines (e.g., TACT908 (2ZEDMA) , 1ZP2MA , 1Z2MAP1O )Indolylaminopropanes (e.g., 1-API, 2-API, 4-API, 5-API (5-IT; PAL-571) , 6-API (6-IT) , 7-API)Iprindole Masupirdine Medmain
Molindone Non-tryptamine triptans (e.g., avitriptan , LY-334370 , naratriptan )
Ondansetron Oxazinopyridoindoles (e.g., IHCH-8134 )Phenethylamines (e.g., phenethylamine , amphetamine )Piperidinylindoles (e.g., BRL-54443 , LY-334370 , naratriptan , sertindole , SN-22 )Pirlindole Pyridinylindoles (e.g., tepirindole )Pyridopyrroloquinoxalines (e.g., IHCH-7113 , IHCH-7079 , IHCH-7086 , lumateperone , deulumateperone , ITI-1549 )Pyrrolylethylamines (e.g., 2-pyrrolylethylamine (NEA) , 3-pyrrolylethylamine (3-NEA) , 3-pyrrolylpropylamine )Pyrrolopyridinylethylamines (e.g., WAY-208466 )Quinolinylethylamines (e.g., mefloquine )Ro60-0213 Selisistat Tetrahydropyridinylindoles (e.g., EMD-386088 , LY-367265 , RU-24,969 )Tetrahydropyridinylpyrrolopyridines (e.g., (R )-69 (3IQ) , (R )-70, CP-94253 )Tetrindole Tipindole Zilpaterol (RU-42173)
See also: PhenethylaminesErgolines and lysergamides Psychedelics