Pinoxaden
 | |
| Names | |
|---|---|
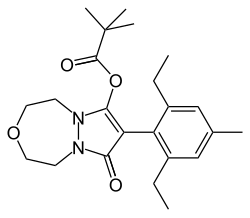
| IUPAC name
8-(2,6-diethyl-p-tolyl)-1,2,4,5-tetrahydro-7-oxo-7H-pyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl 2,2-
dimethylpropionate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.163.258 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H32N2O4 | |
| Molar mass | 400.519 g·mol−1 |
| Moderate[1] | |
| Hazards | |
| GHS labelling:[1] | |
| |
| Warning | |
| H302, H317, H319, H332, H335, H361, H400, H412 | |
| P203, P261, P264, P264+P265, P270, P271, P272, P273, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P318, P319, P321, P330, P333+P317, P337+P317, P362+P364, P391, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
LC50 (median concentration)
|
>5220 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Pinoxaden is a post-emergent phenylpyrazoline[2] herbicide used to control grass weeds in wheat and barley. It is used in the UK and Europe. Japan has a high MRL (maximum residue level) and may use pinoxaden too.[1]
Pinoxaden's HRAC classification is Group A (Australia and global), or Group 1 (numeric). Group A herbicides inhibit acetyl CoA carboxylase, (ACCase).[2]
Application
Pinoxaden may be applied by air or ground equipment at a rate of 15-30 g/Ha (active ingredient), with a spray volume of 50 to 110 L/Ha, or 20-30 L/Ha if applied aerially.[1]
Pinoxaden can control wild oats, paradoxa grass and canary grass.
Cloquintocet-mexyl is similar to pinoxaden and included in many pinoxaden formulations.[1]
Safety
Pinoxden is practically non-toxic, with acute LD50s of over 5000 mg/kg (oral) and over 2000 mg/kg (skin contact). It is a severe eye irritant, but not a skin irritant.[1]
Dogs have been tested with doses of 250 to 1000 mg/kg/day of pinoxaden, there were no visible side effects except a large amount of barfing. A NOEL is not well established, as even at 1000 mg/kg, no effect was observed. Vomiting, apparently does not count as a side-effect.[1]
Rats given up to 1000 mg/kg/day (for 28 days) had reduced weight gain, higher neutrophil, lymphocyte, plasma urea, bilirubin and creatinine levels in males only. Both sexes had increased phosphate, increased piss and ketone production, higher liver and kidney weights, remal tubular atrophy and decreased chloride levels.[1]
Based on subchronic studies, the NOEL is 10 to 100 mg/kg/day.[1]
Environmental fate
In plants, pinoxaden is rapidly metabolised. The most common metabolite is undetectable after 21 days, since it will in turn have been entirely metabolised into more products.[1]
Pinoxaden is moderately hydrophilic and has low volatility. Most pinoxaden left after harvest should become associated with the soil and insterstitial water, with some aquatic exposure from spray drift or runoff, but due to the low volatility, no significant atmospheric exposure. Pinoxaden rapidly hydrolyses, especially in basic conditions. Pinoxaden is mobile in soil, but the low application rates (15-30 g/Ha) limit any leaching. Pinoxaden's soil half-life heavily depends on pH, being 17 days at pH 5, down to 5 hours at pH 9, and typically being less than a day due to fast hydrolysis.[1]
It is not expected to bioaccumulate in fish, and is practically non-toxic to birds, and in general to other wildlife, as the inert formulation elements (solvents, surfactants, etc) are more toxic.[1]