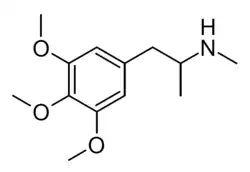
Methyl-TMA, or N-methyl-TMA, also known as N-methyl-3,4,5-trimethoxyamphetamine, is a psychedelic drug of the phenethylamine, amphetamine, and 3C families.[1][2] It is the N-methyl derivative of 3,4,5-trimethoxyamphetamine (TMA) as well as the α,N-dimethyl derivative of mescaline (3,4,5-trimethoxyphenethylamine).[1][2]
Use and effects
N-Methylation of psychedelic phenethylamines has invariably greatly reduced or eliminated their hallucinogenic activity.[3][1][4][5] Examples of this include related compounds like Beatrice (N-methyl-DOM) and methyl-DOB (N-methyl-DOB), which at assessed doses appear to be inactive as psychedelics in humans.[6][1][4][5] According to Alexander Shulgin in his book PiHKAL (Phenethylamines I Have Known and Loved) however, methyl-TMA showed "some mental disturbances" at the highest assessed dose of 240 mg.[1] For comparison, the active dose range of TMA is 100 to 250 mg.[1]
History
Methyl-TMA was first described in the scientific literature by at least 1984.[2][7] It was subsequently described further by Shulgin in PiHKAL in 1991.[1]
See also
References
- ^ a b c d e f g h Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. "Three additional N-methylated homologues of known psychedelics warrant mention, but do not really deserve separate recipes. This is because they have had only the most cursory assaying, which I have learned about by personal correspondence. [...] METHYL-TMA [...] had been run up in several trials to a maximum of 240 [mg], with some mental disturbances mentioned only at this highest level. METHYL-TMA-2 [...] had been tried at up to 120 [mg] without any effects. METHYL-TMA-6 [...] had been tried at up to 30 [mg] and it, too, was apparently without effects. These are reports that I have heard from others, but I have had no personal experience with them. Those that I can describe from personal experience are entered separately as recipes of their own. And there are many, many other N-methyl homologues which have been prepared and characterized in the literature, and have yet to be tasted. So far, however, the only consistent thing seen is that, with N-methylation, the potency of the psychedelics is decreased, but the potency of the stimulants appears to be pretty much maintained."
- ^ a b c Shulgin A, Manning T, Daley P (2011). The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds. Vol. 1. Berkeley: Transform Press. pp. 288, 350, 383, 408. ISBN 978-0-9630096-3-0.
- ^ Nichols DE (2018). Chemistry and Structure-Activity Relationships of Psychedelics. Current Topics in Behavioral Neurosciences. Vol. 36. pp. 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. PMID 28401524.
Although the most active tryptamine hallucinogens are N,N-dialkylated, the phenethylamines generally cannot tolerate even a single N-substitution. Even small groups such as methyl or ethyl (see Table 2) abolish their hallucinogenic activity.
- ^ a b Shulgin AT (1980). "Hallucinogens". In Burger A, Wolf ME (eds.). Burger's Medicinal Chemistry. Vol. 3 (4 ed.). New York: Wiley. pp. 1109–1137. ISBN 978-0-471-01572-7. OCLC 219960627.
Of all the variously substituted phenylisopropylamines that have been N-methylated and titrated in man (including the homologs of TMA-2, 2,5-DMA, DOM, and DOB: 60.22b, 60.22i, 60.22aa, and 60.22ff, respectively), it is only the methylenedioxy compound 60.23a that has maintained quantitative potency (94). As with mescaline itself, dimethylation of this compound eliminates any central action.
- ^ a b Jacob P, Shulgin AT (1994). "Structure-Activity Relationships of the Classic Hallucinogens and Their Analogs". In Lin GC, Glennon RA (eds.). Hallucinogens: An Update (PDF). National Institute on Drug Abuse Research Monograph Series. Vol. 146. National Institute on Drug Abuse. pp. 74–91. PMID 8742795. Archived from the original on 13 July 2025.
[MDA] is also remarkable because the N-methyl homolog 3,4 (MDMA) has biological activity, although the nature of its action places it outside of this review. No other phenethylamine hallucinogen retains central activity on N-methylation.
- ^ Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine: von der Struktur zur Funktion [Phenethylamines: From Structure to Function]. Nachtschatten-Science (in German) (1 ed.). Solothurn: Nachtschatten-Verlag. pp. 834–835, 878. ISBN 978-3-03788-700-4. OCLC 858805226.
8.5.26. N-Substitution von 2,4,5-trisubstituierten Phenylalkylaminen: Einerseits wurde der Einfluss von N-Alkyl-, andererseits derjenige von N-Heterogruppen-Substituenten geprüft. Allgemein ist bekannt, dass das Einführen von Alkylsubstituenten am Stickstoff von psychedelischen Phenylalkylaminen eine Abnahme der HT2-Rezeptoraffinitäten zur Folge hat [29, 150, 151]. Die Wirkungsabschwächung konnte mit den potenten Substanzen DOB (2) und DOM (8) im Menschen bestätigt werden [8]: N-Methyl-DOM (316; BEATRICE) und METHYL-DOB (317) erwiesen sich im Vergleich zu den beiden unmethylierten Verbindungen als massiv weniger aktiv; die aktive Dosis wurde dabei noch nicht eruiert. METHYL-DOET (318; DOETM) erwies sich bei einer Dosierung von 18mg bereits als deutlich aktiv [140]; die Wirkungen wurden im Vergleich zu DOET (14) als ruhiger und angenehmer beschrieben. [...] 318; METHYL-DOET, 18mg, 8-10h. [...] [140] P. Rausch. Persönliche Mitteilung, 2009.
- ^ Clark C (1 October 1984). "The Identification of Methoxy-N-Methylamphetamines". Journal of Forensic Sciences. 29 (4): 1056–1071. doi:10.1520/JFS11772J. ISSN 0022-1198.
External links
|
|---|
| | No ring subs. | |
|---|
| 4-Hydroxytryptamines | |
|---|
| 5-Hydroxytryptamines | |
|---|
| 5-Methoxytryptamines | |
|---|
| Other ring subs. |
- 2,N,N-TMT
- 4,N,N-TMT
- 5-Bromo-DMT
- 5-Chloro-DMT
- 5-Fluoro-DMT
- 5-N,N-TMT
- 7,N,N-TMT
- 5-MeO-2,N,N-TMT
- 5-MeO-4,N,N-TMT
- 6-Fluoro-DMT
- Bretisilocin (GM-2505; 5-fluoro-MET)
|
|---|
| α-Alkyltryptamines |
- 5-Methoxy-α-alkyltryptamines: 5-MeO-AET
- α,N,N-TMT (α-Me-DMT; Alpha-N)
- 5-MeO-AMT (α,O-DMS; Alpha-O)
- α,N,O-TMS (5-MeO-α,N-DMT)
- α,N,N,O-TeMS (5-MeO-α,N,N-TMT)
|
|---|
| Others | |
|---|
|
- Ergolines/lysergamides (e.g., LSD)
- β-Carbolines and Harmala alkaloids (e.g., harmine, harmaline, 6-methoxyharmalan)
- Iboga alkaloids (e.g., 18-MAC, 18-MC, coronaridine, ibogaine, ibogamine, ME-18-MC, noribogaine, tabernanthine, voacangine)
- Ibogalogs (e.g., ibogainalog)
- O-Methylnordehydrobufotenine
- Partial ergolines (e.g., NDTDI, RU-28306, CT-5252)
- Piperidinylethylindoles (e.g., pip-T)
- Pyrrolidinylethylindoles (e.g., pyr-T, 5-MeO-pyr-T)
- Pyrrolidinylmethylindoles (e.g., MPMI, 4-HO-MPMI (lucigenol), 5-MeO-MPMI)
|
|---|
|
- Benzofurans (e.g., 5-MeO-DiBF, dimemebfe (5-MeO-BFE), mebfap)
- Benzothiophenes (e.g., 3-APBT)
- Indazolethylamines (e.g., AL-38022A, O-methyl-AL-34662)
- Indenylethylamines (e.g., C-DMT)
- Isotryptamines (e.g., 6-MeO-isoDMT, Ro60-0175)
- MYCO-005
- Quinolinylethylamines (e.g., mefloquine)
|
|---|
|
|---|
| | |
|---|
|
- Others: 2C-G-x (e.g., 2C-G-3, 2C-G-5)
- β-Keto-2C-B (βk-2C-B)
- β-Keto-2C-I (βk-2C-I)
- β-Methyl-2C-B (BMB)
- (e.g., BOB, BOD, BOH-2C-B)
- (e.g., HOT-2, HOT-7, HOT-17)
- N-Ethyl-2C-B
- (e.g., 2CD-2-ETO, 2CD-5-ETO, 2CE-5-ETO, 2CE-5iPrO, 2CT2-5-ETO, ASR-2001 (2CB-5PrO))
|
|---|
| |
|---|
| |
|---|
| |
|---|
| |
|---|
| |
|---|
| |
|---|
| |
|---|
| Others |
- 2-TOET
- 2-TOM
- 25B-NAcPip
- 4-HA
- 5-TOET
- 5-TOM
- Benzofurans (e.g., 5-APB, 5-APDB, 6-APB, 6-APDB, F, F-2, F-22)
- Benzothiophenes (e.g., 5-APBT, 6-APBT)
- CT-5172
- DMAs (e.g., 2,4-DMA, 3,4-DMA)
- Fenfluramine
- MMA (3-MeO-4-MA)
- Norfenfluramine
- (e.g., 25D-NM-NDEAOP, DOB-NDEPA, DOI-NDEPA, DOM-NDEPA, DOTFM-NDEPA, M-NDEPA, TMA-2-NDEPA)
- PMA (4-MA)
- (e.g., TMA-3, TMA-4, TMA-5)
- TOMSO
- ZDCM-04
|
|---|
|
- 1-Aminomethylindanes (e.g., 2CB-Ind, jimscaline)
- 2-Aminoindanes (e.g., DOM-AI)
- 3-Phenylpiperidines (e.g., LPH-5, LPH-48)
- Benzazepines (e.g., lorcaserin)
- Benzocyclobutenes (e.g., 2CBCB-NBOMe, TCB-2, tomscaline)
- Benzoxepins (e.g., BBOX, IBOX, TFMBOX)
- DMBMPP (juncosamine)
- Ergolines/lysergamides (e.g., LSD)
- Glaucine
- Partial ergolines (e.g., NDTDI, DEIMDHPCA, DEMPDHPCA, DEMTMPDHPCA, DEMNDHPCA)
- Phenylcyclopropylamines (e.g., DMCPA, TMT)
- Phenyloxazolamines (aminorexes) (e.g., 2C-B-aminorex)
- Pyridopyrroloquinoxalines (e.g., IHCH-7113)
- Z3517967757
- ZC-B
|
|---|
|
|---|
| |
|---|
| Others |
- Arylpiperazines (e.g., 2C-B-PP, 2-NP, mCPP, MK-212, ORG-12962, pCPP, pFPP, quipazine, TFMPP)
- Dihydrobenzoxazines (e.g., efavirenz)
- Phenoxyethylamines (e.g., CT-4719, ORG-37684)
- Pyridopyrroloquinoxalines (e.g., IHCH-7113)
- Quinazolinylethylamines (e.g., RH-34)
|
|---|
| Natural sources |
- Tryptamines: Acacia spp. (e.g., Acacia acuminata, Acacia confusa)
- Ayahuasca and vinho de Jurema (e.g., Psychotria viridis (chacruna), Dipolopterys cabrerana (chaliponga, chacruna), Mimosa tenuiflora (Mimosa hostilis; jurema))
- Brosimum (e.g., Brosimum acutifolium (takini))
- Hallucinogenic snuffs (e.g., Anadenanthera peregrina (yopo, jopo, cohoba, parica, ebene), Anadenanthera colubrina (vilca, cebil))
- Incilius alvarius (Bufo alvarius; Colorado River toad, Sonoran Desert toad; bufo)
- Psilocybin-containing mushrooms (magic mushrooms, shrooms) (e.g., Psilocybe cubensis, Psilocybe mexicana (teonanacatl))
- Lysergamides: Achnatherum robustum (sleepy grass)
- Epichloë spp.
- Ergot (Claviceps) (e.g., Claviceps purpurea, Claviceps paspali)
- Morning glory (Convolvulaceae) seeds (e.g., Ipomoea tricolor (tlitliltzin, badoh negro; Ipomoea violacea), Ipomoea corymbosa (coaxihuitl, ololiúqui; Rivea Corymbosa, Turbina Corymbosa), Argyreia nervosa (Hawaiian baby woodrose; HBWR))
- Periglandula spp. (e.g., Periglandula ipomoeae, Periglandula clandestina)
|
|---|
- See also: Hallucinogens
- Entactogens
- Tryptamines
- Phenethylamines
- Ergolines and lysergamides
- Serotonin receptor modulators
|
|
|---|
| Phenethylamines | |
|---|
| Amphetamines | |
|---|
| Phentermines | |
|---|
| Cathinones | |
|---|
Phenylisobutylamines
(and further-extended) | |
|---|
Catecholamines
(and close relatives) | |
|---|
Cyclized
phenethylamines | | Phenylalkylpyrrolidines | |
|---|
2-Benzylpiperidines
(phenidates) | |
|---|
Phenylmorpholines
(phenmetrazines) | |
|---|
Phenyloxazolamines
(aminorexes) | |
|---|
Isoquinolines and
tetrahydroisoquinolines | |
|---|
| 2-Aminoindanes | |
|---|
| 2-Aminotetralins | |
|---|
| Others / unsorted |
- 1-Aminomethylindanes (e.g., 2CB-Ind, AMMI, bromojimscaline, jimscaline)
- 2-ADN
- 2-Benzhydrylpyrrolidine
- 2C-B-5-hemiFLY-α6 (BNAP)
- 2C-B-PYR
- 2CBecca
- 2CJP
- 2CLisaB
- 2CLisaH
- 3-Benzhydrylmorpholine
- 3-Phenylpiperidines (e.g., 3-phenylpiperidine, 3-PPP, OSU-6162 (PNU-96391), LPH-5, LPH-48, Z3517967757 (Z7757))
- 6-AB
- AL-1095
- Aminochromes (e.g., adrenochrome, adrenolutin)
- Benzazepines (e.g., fenoldopam, lorcaserin, SCHEMBL5334361)
- Benzocyclobutenes (e.g., 2CBCB-NBOMe, bromotomscaline, S33005, TCB-2, tomscaline)
- Benzoxepins (e.g., BBOX, IBOX, TFMBOX)
- Butyltolylquinuclidine
- Camfetamine
- Cypenamine (trans-2-phenylcyclopentylamine)
- Diphenidine
- Diphenylprolinol
- DMBMPP
- Ergolines (e.g., LSD)
- Fencamfamin
- GYKI-52895
- HDMP-29
- Ivabradine
- Methoxphenidine
- Methylmorphenate
- Milnacipran
- MT-45
- 2-Naphthylamine
- Org 6582
- Partial ergolines (e.g., NDTDI, RU-27849, DEIMDHPCA, DEMPDHPCA, DEMPDHPCA-2C-D, RU-27251)
- PF-592,379
- Phenylcyclopropylamines (e.g., DMCPA, TMT, tranylcypromine)
- Phenylpiracetams (e.g., phenylpiracetam, MRZ-9547, RGPU-95)
- Pyridopyrroloquinoxalines (e.g., lumateperone, deulumateperone, IHCH-7079, IHCH-7086, IHCH-7113, ITI-1549)
- Tetrahydrobenzopyranylamines (e.g., CT-5126)
- Tolazoline
- Tricyclics (e.g., AMDA, AMDH, benzoctamine, dizocilpine, SpAMDA)
- ZC-B
|
|---|
|
|---|
| Related compounds |
- 2-Furylethylamine
- 2-Pyrrolylethylamine
- 3-Pyrrolylethylamine
- 3-Pyrrolylpropylamine
- 2-Tetrahydrofurylethylamine
- 4-Benzylpiperidine
- 7-AB
- Alkylamines (e.g., 1,3-DMBATooltip 1,3-dimethylbutylamine, 1,4-DMAATooltip 1,4-dimethylamylamine, heptaminol, iproheptine, isometheptene, methylhexanamine/1,3-DMAA, octodrine, oenethyl, tuaminoheptane)
- Benzylamines (e.g., benzylamine, α-methylbenzylamine, MDM1EA, ALPHA, M-ALPHA, pargyline)
- Benzylpiperazines (e.g., benzylpiperazine, MDBZP, fipexide)
- Cyclohexylaminopropanes (e.g., propylhexedrine, norpropylhexedrine)
- Cyclopentylaminopropanes (e.g., isocyclamine, cyclopentamine)
- Phenoxyethylamines (e.g., 3,4,5-trimethoxyphenoxyethylamine, CT-4719, ORG-37684)
- Phenylalkenylamines (e.g., phenylbutenamine)
- Phenylalkynylamines (e.g., phenylbutynamine)
- Phenylpiperazines (e.g., 1-phenylpiperazine, mCPPTooltip meta-chlorophenylpiperazine, TFMPPTooltip trifluoromethylphenylpiperazine, oMPPTooltip ortho-methylphenylpiperazine, pFPPTooltip para-fluorophenylpiperazine, pMeOPPTooltip para-methoxyphenylpiperazine)
- Phenylpropylamines (e.g., phenylpropylamine, homo-MDA, homo-MDMA)
- Thienylaminopropanes (thiopropamines) (e.g., thiopropamine, methiopropamine, thiothinone)
|
|---|
- See also: Tryptamines
- Ergolines and lysergamides
- Stimulants
- Entactogens
- Psychedelics
|