Huprine Y
 | |
| Names | |
|---|---|
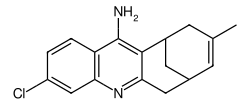
| IUPAC name
7-chloro-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]heptadeca-2,4(9),5,7,10,14-hexaen-3-amine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C17H17ClN2 | |
| Molar mass | 284.79 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Huprine Y is an anticholinesterase compound. According to research, it could potentially have usefulness in management of certain diseases such as Alzheimer's disease.[1][2]
Chemistry
At the chemical level, huprine Y has a very similar structure to huprine X[3][4], another AChE inhibitor[5]. This chemical similarity could explain their similar mechanisms of action.
Mechanism of action
As an anticholinesterase compound, its main mechanism of action is inhibition of the acetylcholine esterase enzyme, which hydrolyses acetylcholine[6]. In other words, Huprine Y reduces the action of the AChE enzyme, resulting in elevated levels of acetylcholine. Like other similar compounds, it appears to have strong affinity when binding to the enzyme.[7][8]
References
- ^ Galdeano, Carles; Viayna, Elisabet; Sola, Irene; Formosa, Xavier; Camps, Pelayo; Badia, Albert; Clos, M. Victòria; Relat, Júlia; Ratia, Míriam; Bartolini, Manuela; Mancini, Francesca; Andrisano, Vincenza; Salmona, Mario; Minguillón, Cristina; González-Muñoz, Gema C. (2012-01-26). "Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer's and prion diseases". Journal of Medicinal Chemistry. 55 (2): 661–669. doi:10.1021/jm200840c. ISSN 1520-4804. PMID 22185619.
- ^ Viayna, Elisabet; Coquelle, Nicolas; Cieslikiewicz-Bouet, Monika; Cisternas, Pedro; Oliva, Carolina A.; Sánchez-López, Elena; Ettcheto, Miren; Bartolini, Manuela; De Simone, Angela; Ricchini, Mattia; Rendina, Marisa; Pons, Mégane; Firuzi, Omidreza; Pérez, Belén; Saso, Luciano (2021-01-14). "Discovery of a Potent Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase with Antioxidant Activity that Alleviates Alzheimer-like Pathology in Old APP/PS1 Mice". Journal of Medicinal Chemistry. 64 (1): 812–839. doi:10.1021/acs.jmedchem.0c01775. hdl:11585/790319. ISSN 1520-4804. PMID 33356266.
- ^ PubChem. "Huprine Y". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-07-29.
- ^ PubChem. "Huprine X". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-07-29.
- ^ Camps, P.; Cusack, B.; Mallender, W. D.; El Achab, R. E.; Morral, J.; Muñoz-Torrero, D.; Rosenberry, T. L. (February 2000). "Huprine X is a novel high-affinity inhibitor of acetylcholinesterase that is of interest for treatment of Alzheimer's disease". Molecular Pharmacology. 57 (2): 409–417. doi:10.1016/S0026-895X(24)23214-4. ISSN 0026-895X. PMID 10648652.
- ^ Dvir, Hay; Silman, Israel; Harel, Michal; Rosenberry, Terrone L.; Sussman, Joel L. (2010-09-06). "Acetylcholinesterase: from 3D structure to function". Chemico-Biological Interactions. 187 (1–3): 10–22. Bibcode:2010CBI...187...10D. doi:10.1016/j.cbi.2010.01.042. ISSN 1872-7786. PMC 2894301. PMID 20138030.
- ^ Camps, Pelayo; Formosa, Xavier; Muñoz-Torrero, Diego; Petrignet, Julien; Badia, Albert; Clos, M. Victoria (2005-03-24). "Synthesis and pharmacological evaluation of huprine-tacrine heterodimers: subnanomolar dual binding site acetylcholinesterase inhibitors". Journal of Medicinal Chemistry. 48 (6): 1701–1704. doi:10.1021/jm0496741. ISSN 0022-2623. PMID 15771413.
- ^ Ronco, Cyril; Carletti, Eugénie; Colletier, Jacques-Philippe; Weik, Martin; Nachon, Florian; Jean, Ludovic; Renard, Pierre-Yves (2012). "Huprine Derivatives as Sub-Nanomolar Human Acetylcholinesterase Inhibitors: From Rational Design to Validation by X-ray Crystallography". ChemMedChem. 7 (3): 400–405. doi:10.1002/cmdc.201100438. ISSN 1860-7187. PMID 22052791.