Canbisol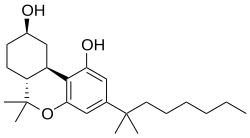 |
 |
|
| Drug class | Cannabinoid |
|---|
| ATC code | |
|---|
|
(6aR,9R,10aR)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,8,9,10,10a-hexahydrobenzo[c]chromene-1,9-diol
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C24H38O3 |
|---|
| Molar mass | 374.565 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
Oc1cc(C(C)(C)CCCCCC)cc(c1C2C3)OC(C)(C)C2CCC3O
|
InChI=1S/C24H38O3/c1-6-7-8-9-12-23(2,3)16-13-20(26)22-18-15-17(25)10-11-19(18)24(4,5)27-21(22)14-16/h13-14,17-19,25-26H,6-12,15H2,1-5H3/t17?,18-,19-/m0/s1  N NKey:UEKGZFCGRQYMRM-MNNMKWMVSA-N  N N
|
 N N Y (what is this?) (verify) Y (what is this?) (verify) |
Canbisol (Nabidrox) is a synthetic cannabinoid derivative that is the dimethylheptyl homologue of 9-nor-9β-hydroxyhexahydrocannabinol (HHC). It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.1 nM at CB1 and 0.2 nM at CB2.[1] It is mainly used in scientific research, in receptor binding studies to determine the structure and function of the cannabinoid receptors,[2][3][4] but has been made illegal in some countries due to its possible abuse potential as a cannabinomimetic drug.[5]
See also
References
- ^ Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, et al. (September 1997). "Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase". Journal of Medicinal Chemistry. 40 (20): 3228–33. doi:10.1021/jm970126f. PMID 9379442.
- ^ Rhee MH, Nevo I, Bayewitch ML, Zagoory O, Vogel Z (December 2000). "Functional role of tryptophan residues in the fourth transmembrane domain of the CB(2) cannabinoid receptor". Journal of Neurochemistry. 75 (6): 2485–91. doi:10.1046/j.1471-4159.2000.0752485.x. PMID 11080201. S2CID 18339666.
- ^ Rhee MH (September 2002). "Functional role of serine residues of transmembrane dopamin VII in signal transduction of CB2 cannabinoid receptor". Journal of Veterinary Science. 3 (3): 185–91. doi:10.4142/jvs.2002.3.3.185. PMID 12514330.
- ^ Zhang R, Hurst DP, Barnett-Norris J, Reggio PH, Song ZH (July 2005). "Cysteine 2.59(89) in the second transmembrane domain of human CB2 receptor is accessible within the ligand binding crevice: evidence for possible CB2 deviation from a rhodopsin template". Molecular Pharmacology. 68 (1): 69–83. doi:10.1124/mol.104.007823. PMID 15840841. S2CID 6488891.
- ^ The Misuse of Drugs Act 1971 (Amendment) Order 2009
|
|---|
Phytocannabinoids
(comparison) | | Cannabibutols | |
|---|
| Cannabichromenes | |
|---|
| Cannabicyclols | |
|---|
| Cannabidiols | |
|---|
| Cannabielsoins | |
|---|
| Cannabigerols | |
|---|
| Cannabiphorols | |
|---|
| Cannabinols |
- CBN
- CBNA
- CBN-C1
- CBN-C2
- CBN-C4
- CBNM
- CBND
- CBNP
- CBVD
|
|---|
| Cannabitriols | |
|---|
| Cannabivarins | |
|---|
| Delta-3-tetrahydrocannabinols | |
|---|
| Delta-4-tetrahydrocannabinols | |
|---|
| Delta-7-tetrahydrocannabinols | |
|---|
| Delta-8-tetrahydrocannabinols | |
|---|
| Delta-9-tetrahydrocannabinols | |
|---|
| Delta-10-Tetrahydrocannabinols | |
|---|
| Delta-11-Tetrahydrocannabinols | |
|---|
| Miscellaneous cannabinoids | |
|---|
| Active metabolites | |
|---|
|
|---|
| Endocannabinoids | |
|---|
Synthetic
cannabinoid
receptor
agonists /
neocannabinoids | Classical cannabinoids
(dibenzopyrans) | |
|---|
Non-classical
cannabinoids | |
|---|
| Adamantoylindoles | |
|---|
| Benzimidazoles | |
|---|
| Benzoylindoles | |
|---|
| Cyclohexylphenols | |
|---|
| Eicosanoids | |
|---|
Indazole-3-
carboxamides | |
|---|
| Indole-3-carboxamides | |
|---|
| Indole-3-carboxylates | |
|---|
| Naphthoylindazoles | |
|---|
| Naphthoylindoles | |
|---|
| Naphthoylpyrroles | |
|---|
| Naphthylmethylindenes | |
|---|
| Naphthylmethylindoles | |
|---|
| Phenylacetylindoles | |
|---|
| Pyrazolecarboxamides | |
|---|
Tetramethylcyclo-
propanoylindazoles | |
|---|
Tetramethylcyclo-
propanoylindoles | |
|---|
| Others | |
|---|
|
|---|
| Allosteric CBRTooltip Cannabinoid receptor ligands | |
|---|
Endocannabinoid
enhancers
(inactivation inhibitors) | |
|---|
Anticannabinoids
(antagonists/inverse
agonists/antibodies) | |
|---|
|
|
|---|
Receptor
(ligands) | | CB1Tooltip Cannabinoid receptor type 1 | Agonists
(abridged,
full list) | |
|---|
| Inverse agonists | |
|---|
| Antagonists | |
|---|
|
|---|
| CB2Tooltip Cannabinoid receptor type 2 | | Agonists |
- 2-AG
- 2-AGE (noladin ether)
- 3,3'-Diindolylmethane
- 4-O-Methylhonokiol
- α-Amyrin · β-Amyrin
- A-796,260
- A-834,735
- A-836,339
- AM-1172
- AM-1221
- AM-1235
- AM-1241
- AM-2232
- Anandamide
- AZ-11713908
- Cannabinol
- Caryophyllene
- CB-13
- CBS-0550
- CP 55,940
- GW-405,833 (L-768,242)
- GW-842,166X
- HU-308
- JTE 7-31
- JWH-007
- JWH-015
- JWH-018
- JWH-73
- JWH-133
- L-759,633
- L-759,656
- Lenabasum (anabasum)
- Magnolol
- MDA-19
- Nabitan
- NADA
- Olorinab (APD-371)
- PF-03550096
- S-444,823
- SER-601
- Serinolamide A
- UR-144
- Tedalinab
- THC (dronabinol)
- THCV
- Tetrahydromagnolol
- Virodhamine
|
|---|
| Antagonists | |
|---|
|
|---|
NAGly
(GPR18) | |
|---|
| GPR55 | |
|---|
| GPR119 | |
|---|
|
|---|
Transporter
(modulators) | | eCBTsTooltip Endocannabinoid transporter | |
|---|
|
|---|
Enzyme
(modulators) | | FAAHTooltip Fatty acid amide hydrolase | |
|---|
| MAGL | |
|---|
| ABHD6 |
- Inhibitors: JZP-169
- JZP-430
- KT182
- KT185
- KT195
- KT203
- LEI-106
- ML294
- ML295
- ML296
- UCM710
- WWL-70
|
|---|
| ABHD12 | |
|---|
|
|---|
| Others |
- Others: 2-PG (directly potentiates activity of 2-AG at CB1 receptor)
- ARN-272 (FAAH-like anandamide transporter inhibitor)
|
|---|
- See also
- Receptor/signaling modulators
- Cannabinoids (cannabinoids by structure)
|

