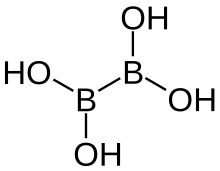
Tetrahydroxydiboron
 | |
| Names | |
|---|---|
| IUPAC name
Hypoboric acid[1]
| |
| Other names
(Dihydroxyboranyl)boronic acid
Hypoboric acid Hypodiboric acid Sub-boric acid (Unterborsäure in German) 1,1,2,2-Tetrahydroxydiborane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.222.662 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| B2H4O4 | |
| Molar mass | 89.65 g·mol−1 |
| Appearance | White powder |
| Density | 1.657 |
| very soluble | |
| Solubility | ethanol, DMF, DMSO, DMA |
| Structure | |
| monoclinic P21/c | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
125.46 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−1410.43 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
| H302, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Related compounds | |
Related compounds
|
Diborane Diboron tetrafluoride Bis(pinacolato)diboron |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Tetrahydroxydiboron is a reagent which has several uses in organic synthesis[2], notably as a precursor for boronic acids used in Suzuki-Miyaura couplings [3]
Synthesis
The reaction of boron trichloride with alcohols was reported in 1931, and was used to prepare dimethoxyboron chloride, B(OCH3)2Cl.[4] Egon Wiberg and Wilhelm Ruschmann used it to prepare tetrahydroxydiboron by first introducing the boron–boron bond by reduction with sodium and then hydrolysing the resulting tetramethoxydiboron, B2(OCH3)4, to produce what they termed sub-boric acid.[5] The methanol used in this process can be recycled:
- BCl3 B(OCH3)2Cl B2(OCH3)4 B2(OH)4
Overall: 2 BCl3 + 2 Na + 4 H2O → B2(OH)4 + 2 NaCl + 4 HCl
Reactions
Tetrahydrodiboron can be used in the Miyaura borylation of aryl halides and psuedo-halides to form aryl boronic acids,[6] which are key components in Suzuki-Miyaura couplings.[7] Tetrahydroxydiboron has also been shown to be a reducing agent for nitro groups, providing an alternative to reactions involving hydrogen gas.[8]
Safety
Although tetrahydroxydiboron has been shown not to be shock or friction sensitive in the solid state, differential scanning calorimetry shows the compound decomposes with significant energy release starting at 90oC,[9] which may be related to the dehydration to a polymeric boron(II) oxide.[10] Similar thermal instability is observed in solution with organic solvents like DMF and DMSO.[11] An additional safety consideration when using tetrahydroxydiboron is that upon exposure to water, tetrahydroxydiboron decomposes to form for two equivalents of boric acid and one equivalent of hydrogen gas.[12]
References
- ^ "Hypodiboric acid". IUPAC.
- ^ Zhao, Qiuxia; Liu, Xiang; Astruc, Didier (2023). "Tetrahydroxydiboron (Bis-boric Acid): a Versatile Reagent for Borylation, Hydrogenation, Catalysis, Radical Reactions and H2 Generation". European Journal of Inorganic Chemistry. 26 (11): e202300024. doi:10.1002/ejic.202300024. ISSN 1099-0682.
- ^ Little, Sarah; Trice, Jane (2001). "Tetrahydroxydiboron". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289x.rn01181. ISBN 9780470842898.
- ^ Wiberg, Egon; Sütterlin, Walther (1931). "Zur Kenntnis einiger Verbindungen vom Typus BCl3−n(OR)n. (Über alkoxyl-substituierte Borchloride)" [Notes on some compounds of type BCl3−n(OR)n. (Via alkoxy-substituted boron chlorides)]. Z. anorg. allg. Chem. (in German). 202 (1): 1–21. doi:10.1002/zaac.19312020102.
- ^ Wiberg, Egon; Ruschmann, Wilhelm (1937). "Über eine neue Borsäure ('Unterborsäure'︁) der Formel H4B2O4 und ihre Ester" [On a new boric acid ('Sub-boric acid') of the formula H4B2O4 and its esters]. Ber. Dtsch. Chem. Ges. A/B (in German). 70 (6): 1393–1402. doi:10.1002/cber.19370700636.
- ^ Chow, Wing Kin; Yuen, On Ying; Choy, Pui Ying; So, Chau Ming; Lau, Chak Po; Wong, Wing Tak; Kwong, Fuk Yee (2013-07-15). "A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates". RSC Advances. 3 (31): 12518–12539. doi:10.1039/C3RA22905J. ISSN 2046-2069.
- ^ Chow, Wing Kin; Yuen, On Ying; Choy, Pui Ying; So, Chau Ming; Lau, Chak Po; Wong, Wing Tak; Kwong, Fuk Yee (2013-07-15). "A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates". RSC Advances. 3 (31): 12518–12539. doi:10.1039/C3RA22905J. ISSN 2046-2069.
- ^ Jang, Mingyeong; Lim, Taeho; Park, Byoung Yong; Han, Min Su (2022-01-21). "Metal-Free, Rapid, and Highly Chemoselective Reduction of Aromatic Nitro Compounds at Room Temperature". The Journal of Organic Chemistry. 87 (2): 910–919. doi:10.1021/acs.joc.1c01431. ISSN 0022-3263.
- ^ Zhang, Shasha; Leung, Simon Shun Wang; Vanyo, Dale (2024-07-19). "Thermal Stability of Tetrahydroxydiboron". Organic Process Research & Development. 28 (7): 2854–2861. doi:10.1021/acs.oprd.4c00159. ISSN 1083-6160.
- ^ Wartik, Thomas; Apple, Eugene F. (1955-12-01). "A NEW MODIFICATION OF BORON MONOXIDE". Journal of the American Chemical Society. 77 (23): 6400–6401. doi:10.1021/ja01628a116. ISSN 0002-7863.
- ^ Revu, Omkar; Vasilev, Maksim; Gajula, Praveen; Kalikinidi, Nageswara Rao; Gadi, Madhusudhan Reddy; Zhao, Huiping; Gamage, Shanika M. P.; Hibbert, Graham; Ravikumar, Ongolu; Gummidi, Lalitha; Nasipireddy, Venkatarathnam; Vinodini, Arun; Bietsch, Jonathan; Wang, Zhirui; Brown, Jack D. (2024-10-18). "Development of a Safer Continuous Flow Process for B2(OH)4-Mediated Chemoselective Reduction of Nitroarenes to Anilines". Organic Process Research & Development. 28 (10): 3847–3857. doi:10.1021/acs.oprd.4c00267. ISSN 1083-6160.
- ^ Merritt, Jeremy M.; Borkar, Indrakant; Buser, Jonas Y.; Brewer, Alison Campbell; Campos, Odilon; Fleming, Jeffrey; Hansen, Caoimhe; Humenik, Ashley; Jeffery, Stephen; Kokitkar, Prashant B.; Kolis, Stanley P.; Forst, Mindy B.; Lambertus, Gordon R.; Martinelli, Joseph R.; McCartan, Ciaran (2022-03-18). "Hydrogen Evolution from Telescoped Miyaura Borylation and Suzuki Couplings Utilizing Diboron Reagents: Process Safety and Hazard Considerations". Organic Process Research & Development. 26 (3): 773–784. doi:10.1021/acs.oprd.1c00198. ISSN 1083-6160.