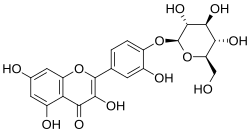
Spiraeoside
Spiraeoside structure
Names
IUPAC name
4′-(β-D -Glucopyranosyloxy)-3,3′,5,7-tetrahydroxyflavone
Systematic IUPAC name
3,5,7-Trihydroxy-2-(3-hydroxy-4-{[(2S ,3R ,4S ,5S ,6R )-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)-4H -1-benzopyran-4-one
Other names
SpiraeosidO -β-D -glucoside
Identifiers
68011
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.039.634
EC Number
UNII
InChI=1S/C21H20O12/c22-6-13-15(26)17(28)19(30)21(33-13)32-11-2-1-7(3-9(11)24)20-18(29)16(27)14-10(25)4-8(23)5-12(14)31-20/h1-5,13,15,17,19,21-26,28-30H,6H2/t13-,15-,17+,19-,21-/m1/s1
N Key: OIUBYZLTFSLSBY-HMGRVEAOSA-N
N InChI=1/C21H20O12/c22-6-13-15(26)17(28)19(30)21(33-13)32-11-2-1-7(3-9(11)24)20-18(29)16(27)14-10(25)4-8(23)5-12(14)31-20/h1-5,13,15,17,19,21-26,28-30H,6H2/t13-,15-,17+,19-,21-/m1/s1
Key: OIUBYZLTFSLSBY-HMGRVEAOBO
c1cc(c(cc1c2c(c(=O)c3c(cc(cc3o2)O)O)O)O)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O
Properties
C 21 H 20 O 12
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Spiraeoside is a chemical compound. It can be isolated from flowers of meadowsweet (Filipendula ulmaria ) or from garden onion (Allium cepa ).[ 1] [ 2]
Spiraeoside is the 4'-O -glucoside of quercetin .
References
^ Williamson, Gary; Plumb, Geoff W.; Uda, Yasushi; Price, Keith R.; Rhodes, Michael J.C. (1996). "Dietary quercetin glycosides: Antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells" . Carcinogenesis . 17 (11): 2385– 7. doi :10.1093/carcin/17.11.2385 PMID 8968052 . ^ Olsson, Marie E.; Gustavsson, Karl-Erik; Vågen, Ingunn M. (2010). "Quercetin and Isorhamnetin in Sweet and Red Cultivars of Onion (Allium cepaL.) at Harvest, after Field Curing, Heat Treatment, and Storage". Journal of Agricultural and Food Chemistry . 58 (4): 2323– 30. doi :10.1021/jf9027014 . PMID 20099844 .
Flavonols and their conjugates
Backbone
Flavonols
Aglycones Conjugates
Glycosides of herbacetin Glycosides of kaempferol
Afzelin (Kaempferol 3-rhamnoside)Astragalin (kaempferol 3-O-glucoside)Kaempferitrin (kaempferol 3,7-dirhamnoside)Juglanin (Kaempferol 3-O-arabinoside)Kaempferol 3-alpha-L-arabinopyranoside
Kaempferol 3-alpha-D-arabinopyranoside
Kaempferol 7-alpha-L-arabinoside
Kaempferol 7-O-glucoside Kaempferol 3-lathyroside
Kaempferol 4'-rhamnoside
Kaempferol 5-rhamnoside
Kaempferol 7-rhamnoside
Kaempferol 7-O-alpha-L-rhamnofuranoside
Kaempferol 3-xyloside
Kaempferol 7-xyloside
Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside)Kaempferol 3-O-rutinoside Sophoraflavonoloside (Kaempferol 3-O-sophoroside)
Trifolin (Kaempferol 3-O-beta-D-galactoside) Glycosides of myricetin Conjugates of quercetin
O -Methylated flavonols
Aglycones Glycosides
of isorhamnetin
Narcissin (Isorhamnetin 3-O-rutinoside)
Isorhamnetin 3-O-glucoside
Tamarixetin 7-rutinoside other
Azalein (Azaleatin 3-O-α-L-rhamnoside)Centaurein (Centaureidin 7-O-glucoside)
Eupalin (Eupalitin 3-0-rhamnoside)Eupatolin (Eupatolitin 3-O-rhamnoside)Jacein (Jaceidin 7-O-glucoside)
Patulitrin (Patuletin 7-O-glucoside
Xanthorhamnin (Rhamnetin glycoside)
Derivative flavonols
Aglycones
Noricaritin
Dihydronoricaritin Glycosides
Pyranoflavonols
Furanoflavonols
Semisynthetic