PR-000608
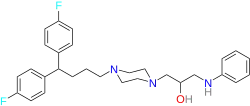 | |
| Clinical data | |
|---|---|
| Other names | PR 000608 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H35F2N3 |
| Molar mass | 463.617 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
PR-608 is a potent dopamine reuptake inhibitor related to vanoxerine. However, the GBR class of agents was known to be derived from diphenhydramine (or more specifically flunamine) as exemplified by S-350 and bears the distinctive benzhydryl-ethyl-ether functional group.[1][2] PR-608 on the other hand belongs to the structurally distinct diphenylbutylpiperazine class of agents (related to the diphenylbutylpiperidine class). Other members of this class include amperozide, lidoflazine, difluanazine,[3][4] FG5865 and FG-5893.[5]
PR-608 was invented and developed in Japan and was patented in the 1990's to Pola Orbis Holdings Inc.[6][7]
PR-608 has been shown in preclinical studies to inhibit dopamine uptake in the central nervous system and may have potential applications in neuropsychiatric and cardiovascular disorders.[8][9][10][6] For example, the compounds were shown to produce a marked increase in locomotor activity (LMA), without a marked decrease in blood pressure. Although PR-608 and vanoxerine showed a similar nanomolar affinity for the DAT, in vivo microdialysis studies showed that PR-608 produced more robust increases in extracellular dopamine than vanoxerine did.
The calcium channel blocking properties of PR-608 might make it useful as a cardiac stimulant for the treatment of heart disease or as a cerebral vasodilator.[11] Alternative applications of this agent include for the treatment of psychostimulant addiction,[12][13][14] neurodegenerative diseases including (but not limited to) Parkinson's disease,[15][16] and as a treatment for depression.[17][18][19][20] Since dopamine regulates the appetite,[21][22][23] PR-608 might also find use for treating binge eating disorder (BED)[24][25][26] as well as treating narcolepsy.[27]
See also
References
- ^ Buzas, A., Champagnac, A., Dehnel, A., Lavielle, G., Pommier, M. (February 1980). "Synthesis and psychoanaleptic properties of new compounds structurally related to diphenhydramine". Journal of Me-dicinal Chemistry. 23 (2): 149–153. doi:10.1021/jm00176a009.
- ^ Buzas, A., Champagnac, A., Dehnel, A., Lavielle, G., Pommier, M. (1 July 1980). "ChemInform Ab-stract: SYNTHESIS AND PSYCHOANALEPTIC PROPERTIES OF NEW COMPOUNDS STRUCTUR-ALLY RELATED TO DIPHENHYDRAMINE". Chemischer Informationsdienst. 11 (26). doi:10.1002/chin.198026225.
- ^ "Difluanazine". PubChem. U.S. National Library of Medicine.
- ^ US 3267104, Hermans HK, Karl-Adolf SW, "1,4-Disubstituted piperazines and diazepines", issued 16 August 1966, assigned to Janssen Pharmaceutica NV
- ^ Hjorth, S., Pettersson, G. (July 1993). "5-HT1A autoreceptor-mediated effects of the amperozide congeners, FG5865 and FG5893, on rat brain 5-hydroxytryptamine neurochemistry in vivo". European Journal of Pharmacology. 238 (2–3): 357–367. doi:10.1016/0014-2999(93)90867-H. PMID 7691622.
- ^ a b US 5391552, Inazu M, Miyata Y, Morimoto T, Yamamoto T, Yoshiko Y, Harada K, Momota Y, Yanagi M, Yokota R, Katoh T, Namiki T, Kimura M, Kawakatsu N, "Diphenylpiperazine derivative and drug for circulatory organ containing the same.", issued 21 February 1995, assigned to Pola Orbis Holdings Inc.
- ^ https://www.po-holdings.co.jp/
- ^ Kimura M, Masuda T, Yamada K, Mitani M, Kubota N, Kawakatsu N, et al. (April 2003). "Syntheses of novel diphenyl piperazine derivatives and their activities as inhibitors of dopamine uptake in the central nervous system". Bioorganic & Medicinal Chemistry. 11 (8): 1621–1630. doi:10.1016/s0968-0896(03)00061-0. PMID 12659747.
- ^ Kimura M, Masuda T, Yamada K, Mitani M, Kubota N, Kawakatsu N, et al. (September 2003). "Novel diphenylalkyl piperazine derivatives with high affinities for the dopamine transporter". Bioorganic & Medicinal Chemistry. 11 (18): 3953–3963. doi:10.1016/s0968-0896(03)00428-0. PMID 12927856.
- ^ Kimura M, Masuda T, Yamada K, Mitani M, Kubota N, Kawakatsu N, et al. (June 2004). "Efficient asymmetric syntheses, determination of absolute configurations and biological activities of 1-[4,4-bis(4-fluorophenyl)butyl]-4-[2-hydroxy-3-(phenylamino)propyl]piperazine as a novel potent dopamine uptake inhibitor in the central nervous system". Bioorganic & Medicinal Chemistry. 12 (11): 3069–3078. doi:10.1016/j.bmc.2004.02.041. PMID 15142566.
- ^ Kimura M, Masuda T, Yamada K, Kubota N, Kawakatsu N, Mitani M, et al. (August 2002). "Novel diphenylalkyl piperazine derivatives with dual calcium antagonistic and antioxidative activities". Bioorganic & Medicinal Chemistry Letters. 12 (15): 1947–1950. doi:10.1016/s0960-894x(02)00322-0. PMID 12113815.
- ^ Rothman RB. High affinity dopamine reuptake inhibitors as potential cocaine antagonists: a strategy for drug development. Life Sci. 1990;46(20):PL17-21. doi: 10.1016/0024-3205(90)90466-5. PMID: 2111866.
- ^ Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999 Jul 29;42(15):2721-36. doi: 10.1021/jm9706729. PMID: 10425082.
- ^ Cohen BM, Carlezon WA Jr. Can't get enough of that dopamine. Am J Psychiatry. 2007 Apr;164(4):543-6. doi: 10.1176/ajp.2007.164.4.543. PMID: 17403963.
- ^ "PR-000608". PatSnap.
- ^ Harada M, Kubota N, Masuda T, Inazu M (January 1996). "P-525: Effects of PR-000608, a novel antiparkinson drug, on MPTP-induced parkinsonism". Japanese Journal of Pharmacology. 71: 190. doi:10.1016/S0021-5198(19)36998-7.
- ^ Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect. 1993;91(2-3):75-109. doi: 10.1007/BF01245227. PMID: 8099801.
- ^ Orr K, Taylor D (2007). "Psychostimulants in the treatment of depression : a review of the evidence". CNS Drugs. 21 (3): 239–57. doi:10.2165/00023210-200721030-00004. PMID 17338594.
- ^ Dunlop BW, Nemeroff CB (March 2007). "The role of dopamine in the pathophysiology of depression". Archives of General Psychiatry. 64 (3): 327–37. doi:10.1001/archpsyc.64.3.327. PMID 17339521.
- ^ Gershon AA, Vishne T, Grunhaus L (January 2007). "Dopamine D2-like receptors and the antidepressant response". Biological Psychiatry. 61 (2): 145–53. doi:10.1016/j.biopsych.2006.05.031. PMID 16934770.
- ^ Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci. 2017 Nov 16;18(12):741-752. doi: 10.1038/nrn.2017.130. PMID: 29142296.
- ^ Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005 May;8(5):555-60. doi: 10.1038/nn1452. PMID: 15856062.
- ^ da Fonseca NKO, Brietzke E. Ultra-processed foods and dopamine: Parsing complexity beyond observed variability. Cell Metab. 2025 Aug 5;37(8):1622-1623. doi: 10.1016/j.cmet.2025.06.008. PMID: 40769124.
- ^ Heal, D. J., Smith, S. L. (June 2022). "Prospects for new drugs to treat binge-eating disorder: Insights from psychopathology and neuropharmacology". Journal of Psychopharmacology. 36 (6): 680–703. doi:10.1177/02698811211032475. PMC 9150143. PMID 34318734.
- ^ Yu, Y., Miller, R., Groth, S. W. (28 January 2022). "A literature review of dopamine in binge eating". Journal of Eating Disorders. 10 (1) 11. doi:10.1186/s40337-022-00531-y. PMC 8796589. PMID 35090565.
- ^ Berner, L. A., Bocarsly, M. E., Hoebel, B. G., & Avena, N. M. (2011). Pharmacological interventions for binge eating: Lessons from animal models, current treatments, and future directions. Current Pharmaceutical Design, 17(12), 1180-1187. https://doi.org/10.2174/138161211795656774
- ^ Toth, B., Burgess, C., Chang, K. (29 May 2023). "0039 Investigating the role of striatal dopamine in sleep and narcolepsy-cataplexy". SLEEP. 46 (Supplement_1): A18. doi:10.1093/sleep/zsad077.0039.