P-1075
 | |
| Clinical data | |
|---|---|
| Other names | P1075 |
| Drug class | ATP-sensitive potassium channel opener; Vasodilator; Antihypertensive agent |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.104 |
| Chemical and physical data | |
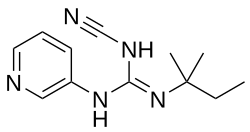
| Formula | C12H17N5 |
| Molar mass | 231.303 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
P-1075 is an ATP-sensitive potassium channel opener which was under development for the treatment of androgenic alopecia (pattern hair loss), arrhythmias, and ischemic heart disorders but was never marketed.[1][2][3][4][5] It has been found to stimulate cultured hair follicles and to promote hair growth in balding stump-tailed macaques.[3][4][5] The drug was being developed by AstraZeneca and LEO Pharma.[1][2] It reached phase 2 clinical trials for alopecia and the preclinical research stage of development for arrhythmia and ischemic heart disorders prior to the discontinuation of its development by 2000.[1][2] In terms of chemical structure, P-1075 is a guanidine derivative and a more potent analogue of pinacidil.[3]
See also
- List of investigational hair loss drugs
- ATP-sensitive potassium channel § Stimulation of hair growth
References
- ^ a b c "P 1075". AdisInsight. 3 November 2000.
- ^ a b c "Delving into the Latest Updates on P-1075 with Synapse". Synapse. 20 July 2025. Retrieved 24 July 2025.
- ^ a b c Buhl AE, Conrad SJ, Waldon DJ, Brunden MN (July 1993). "Potassium channel conductance as a control mechanism in hair follicles". The Journal of Investigative Dermatology. 101 (1 Suppl): 148S – 152S. doi:10.1111/1523-1747.ep12363290. PMID 8326149.
- ^ a b Messenger AG, Rundegren J (February 2004). "Minoxidil: mechanisms of action on hair growth". The British Journal of Dermatology. 150 (2): 186–194. doi:10.1111/j.1365-2133.2004.05785.x. PMID 14996087.
- ^ a b Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S (May 2012). "Minoxidil use in dermatology, side effects and recent patents". Recent Patents on Inflammation & Allergy Drug Discovery. 6 (2): 130–136. doi:10.2174/187221312800166859. PMID 22409453.
Other potassium channel openers, like diazoxide [39, 40] and pinacidil [41] can cause hypertrichosis in humans as well as minoxidil. In balding macaques minoxidil, cromakalin and P-1075 (a pinacidil analogue) stimulate hair growth in about 20 weeks of topical treatment, whereas a fourth potassium channel opener, called RP49356, is not effective [42]. Harmon et al. suggested that minoxidil, diazoxide, cromakalin and pinacidil increased uptake of thymidine in hair growth cultures of mouse vibrissae follicles [43].