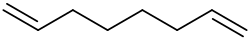
1,7-Octadiene
| Names | |
|---|---|
| Preferred IUPAC name
Octa-1,7-diene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.020.959 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2309 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H14 | |
| Molar mass | 110.200 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.746 g/mL at 25 °C |
| Boiling point | 114–121 °C (237–250 °F; 387–394 K) |
| Hazards | |
| GHS labelling: | |
| |
| Danger | |
| H225, H304, H410, H412 | |
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |
| Related compounds | |
| Isoprene Chloroprene | |
Related compounds
|
Butane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
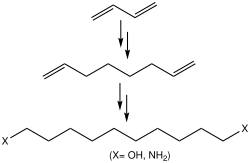
1,7-Octadiene is an organic compound with the formula (CH2=CHCH2CH2)2. It is a colorless liquid that serves as a precursor to specialty polymers. It arises commercially by the dimerization of butadiene in the presence of hydrogen. Some of the 1,6-octadiene is also formed. 1,7-Octadiene can be converted to the diol by hydroformylation followed by hydrogenation of the dialdehyde. In a related process, 1,7-Octadiene undergoes hydrocyanation to give dinitrile, which can be hydrogenated to give 1,10-diaminodecane.[1]
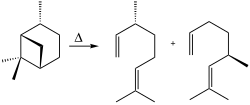
Dimethyloctadienes
Structurally related octadienes bearing two methyl groups are of commercial interest. Such compounds are produced by pyrolysis of pinane, which is abundantly available from terpentine or related wood-derived chemicals.[2]
Research
The diene has also been the subject of many research papers. For example, with ethylene it undergoes a cross-enyne metathesis Diels–Alder reaction.[3] It undergoes ring-closing metathesis to give cyclooctene.[4] Plasma polymerized 1,7-octadiene films deposited on silica can produce particles with tuned hydrophobicity.[5]
References
- ^ Dahlmann, Marc; Grub, Joachim; Löser, Eckhard (2011). "Butadiene". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–24. doi:10.1002/14356007.a04_431.pub2. ISBN 978-3-527-30673-2.
- ^ Sagorin, Gilles; Cazeils, Emmanuel; Basset, Jean-François; Reiter, Maud (2021). "From Pine to Perfume". CHIMIA. 75 (9): 780–787. doi:10.2533/chimia.2021.780. PMID 34526184.
- ^ Fustero, S; Bello, P; Miró, J; Simón, A; del Pozo, C (27 August 2012). "1,7-octadiene-assisted tandem multicomponent cross-enyne metathesis (CEYM)-Diels-Alder reactions: a useful alternative to Mori's conditions". Chemistry: A European Journal. 18 (35): 10991–7. doi:10.1002/chem.201200835. PMID 22851514.
- ^ Weskamp, Thomas; Schattenmann, Wolfgang C.; Spiegler, Michael; Herrmann, Wolfgang A. (1998). "A Novel Class of Ruthenium Catalysts for Olefin Metathesis". Angewandte Chemie International Edition. 37 (18): 2490–2493. doi:10.1002/(sici)1521-3773(19981002)37:18<2490::aid-anie2490>3.0.co;2-x. PMID 29711340.
- ^ Akhavan, Behnam; Jarvis, Karyn; Majewski, Peter (November 2013). "Tuning the hydrophobicity of plasma polymer coated silica particles". Powder Technology. 249: 403–411. doi:10.1016/j.powtec.2013.09.018.
External links
- "1,7-Octadiene". Sigma-Aldrich. Retrieved 20 April 2016.