Miravirsen
 | |
| Clinical data | |
|---|---|
| Other names | SPC3649 |
| Routes of administration | Intravenous or subcutaneous injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C156H195N49O83P14S14 |
| Molar mass | 4967.01 g·mol−1 |
| |
| |
Miravirsen (INN; codenamed SPC3649) is an experimental drug for the treatment of hepatitis C, being developed by Santaris Pharma. As of 2017 it was in Phase II clinical trials.[1]
Miravirsen had been given by subcutaneous injection in early clinical trials as of 2017.[1] It is antisense to a human microRNA called miR-122. miR-122 ferries an argonaute protein to 5'-UTR region of viral RNA, where it binds, protecting the RNA from being destroyed by normally present nucleases; by binding to miR-122, miravirsen removes that protection and the virus RNA can be destroyed.[1] There is some evidence that the 5'-UTR region mutates under repeated exposure to miravirsen.[1]
Chemistry
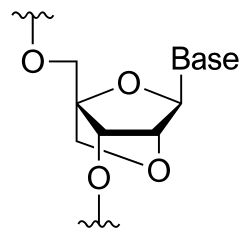
Miravirsen is a modified antisense oligonucleotide consisting of a chain of 15 nucleotides, the base sequence of which is designed to selectively bind to miR-122.[1][2] Seven of the 15 sugar units are deoxyriboses, and the other eight are riboses with an additional bridge between the 2' oxygen and the 4' carbon atoms; this makes miravirsen a locked nucleic acid gapmer. Furthermore, miravirsen uses phosphorothioate linkages instead of the phosphate linkages in natural oligonucleotides to make it stable towards degradation by nucleases.[2]
The complete base sequence is
mC*-dC-A*-dT-dT-G*-mU*-dC-dA-mC*-dA-mC*-dT-mC*-mC*[d= 2'-deoxy,m= 5-methyl,*= 2'-O,4'-C-methylene, i.e. bridged or "locked" sugar]
with 3'→5' phosphorothioate linkages.[2]