Iron bis(diethyldithiocarbamate)
2_dimer.svg.png) | |
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C20H40Fe2N4S8 | |
| Molar mass | 704.74 g·mol−1 |
| Appearance | red solid |
| Density | 1.457 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
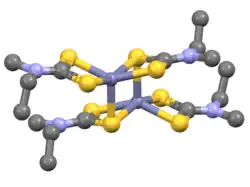
Iron bis(diethyldithiocarbamate) is a coordination complex with the formula [Fe(S2CNEt2)2]2 where Et = ethyl (C2H5). A red solid, iron bis(diethyldithiocarbamate) is representative of several ferrous dithiocarbamates with diverse substituents in place of ethyl. In terms of structure, the species is dimeric, consisting of a pair of pentacoordinate iron(II) centers. It is structurally related to zinc bis(dimethyldithiocarbamate).[1]
Reactions
The complex reacts with a variety of reagents with concomitant formation of mono-iron derivatives. It adds 9,10-phenanthroline (phen) to give the blue-octahedral complex Fe(S2CNEt2)2(phen). 3,4-Bis(trifluoromethyl)-1,2-dithiete reacts to give the Fe(IV) dithiolene complex Fe(S2CNEt2)2(S2C2(CF3)2).[2] Nitric oxide and carbon monoxide add to give the nitrosyl complex Fe(S2CNEt2)2NO and the carbonyl complex Fe(S2CNEt2)2(CO)2, respectively.
Related compounds
References
- ^ Ileperuma, Oliver A.; Feltham, Robert D. (1975). "Crystal and Molecular Structure of Iron(II) Bis(diethyldithiocarbamate)". Inorganic Chemistry. 14 (12): 3042–3045. doi:10.1021/ic50154a037.
- ^ Coucouvanis, Dimitri (1979). "The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes, 1968–1977". Progress in Inorganic Chemistry. Vol. 26. pp. 301–469. doi:10.1002/9780470166277.ch5. ISBN 9780470166277.