Focused ultrasound
| High-intensity focused ultrasound | |
|---|---|
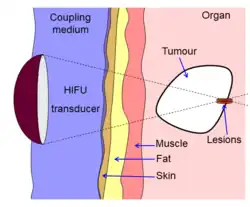 Diagram showing how HIFU can be used to destroy tissue in the body. An acoustic lens is used to focus sound to a small point in the body. The sound propagates through many layers of tissue. Because of the focal gain, only tissue at the focus is destroyed. | |
| Other names | Magnetic-resonance-guided focused ultrasound surgery (MRgFUS), focused ultrasound surgery (FUS), MR-guided focused ultrasound ablation |
High-intensity focused ultrasound (HIFU), or MR-guided focused ultrasound surgery (MR-guided focused ultrasound ablation), is an incisionless therapeutic technique[1] that uses non-ionizing ultrasonic waves to heat or ablate tissue. HIFU can be used to increase the flow of blood or lymph or to destroy tissue, such as tumors, via thermal and mechanical mechanisms. Given the prevalence and relatively low cost of ultrasound generation mechanisms, the premise of HIFU is that it is expected to be a non-invasive and low-cost therapy that can at least outperform care in the operating room.
The technology is different from that used in ultrasonic imaging, though lower frequencies and continuous, rather than pulsed, waves are used to achieve the necessary thermal doses. However, pulsed waves may also be used if mechanical rather than thermal damage is desired. Acoustic lenses are often used to achieve the necessary intensity at the target tissue without damaging the surrounding tissue. The ideal pattern diagram is the beam-focusing of a magnifying glass of sunlight; only the focal point of the magnifying glass has high temperature.
HIFU is combined with other imaging techniques such as medical ultrasound or MRI to enable guidance of the treatment and monitoring.
History
Studies on localized prostate cancer showed that, after treatment, progression-free survival rates were high for low- and intermediate- risk patients with recurrent prostate cancer.[2]
In 2009, the Insightec ExAblate 2000 was the first MRgFUS system to obtain FDA market approval, applying US 5,247,935[3]
In 2016, the US Food and Drug Administration (FDA) approved Insightec's Exablate system to treat essential tremor.[4] Treatment for other thalamocortical dysrhythmias and psychiatric conditions are under investigation.[5]
Medical uses
There is no accepted boundary between HIFU and other forms of therapeutic ultrasound. In some literature, HIFU refers to the high levels of energy required to destroy tissue through ablation or cavitation, although it is also sometimes used to describe lower intensity applications such as occupational and physical therapy.
Either way, HIFU is used to non-invasively heat or ablate tissue deep in the body without an incision.[1] The main applications are the destruction of tissue caused by hyperthermia, increasing perfusion and physical therapy. It later found use to treat tumors of the liver, with clinical trials underway for other sites,[6] as well as musculoskeletal conditions.[7]
Neurological disorders

One of the first applications of HIFU was for Parkinson's disease in the 1940s. Although ineffective at the time, HIFU has the capacity to lesion pathology. Focused ultrasound is approved in Israel, Canada, Italy, Korea and Russia to treat essential tremor,[8] neuropathic pain,[9] and Parkinsonian tremor.[10] This approach enables treatment of the brain without an incision or radiation.
Cancers
HIFU applied to cancers can disrupt the tumor microenvironment and trigger an immune response, as well as possibly enhance the efficacy of immunotherapy.[11][12]
Prostate
HIFU may be effective for treating prostate cancer.[13][14][15]
Liver
HIFU has been studied in liver cancer and many studies report a high response rate and positive outcome.[16] During the treatment of metastasized liver cancer with HIFU, immune responses have been observed in locations distant from the focal region.[17] A 2024 clinical trial of histotripsy on liver tumors, researchers reported a 95% success rate.[18] Clinical trials are underway for treating tumors of the pancreas and kidney.[19]
Histotripsy
Histotripsy is a form of non-thermal HIFU for use on the liver that shows some therapeutic potential.[20] Histotripsy mechanically destroys tissue through cavitation.[21]
Kidney stones
Focused ultrasound may be used to dissolve kidney stones by lithotripsy.
Cataracts
Ultrasound may be used to treat cataracts by phacoemulsification.
Mechanism
Multiple HIFU beams are precisely focused on a small region of diseased tissue to locally deposit high levels of energy. Focused ultrasound can generate localized heating. Focusing can be guided by Magnetic Resonance Imaging (MRgFUS). These procedures generally use lower frequencies than diagnostic ultrasound (0.7 to 2 MHz), but the higher frequency means lower focusing energy.
Temperature
The temperature of tissue at the focus can be increased to between 65 and 85 °C. This induces coagulative necrosis, destroying the tissue. Tissue heated above 60 °C for longer than 1 second becomes irreversibly damaged.[22] Each sonication (individual ultrasound energy deposition) treats a precisely defined portion of tissue. Multiple sonications cover a larger area, creating a volume of incompressible material, such as tap water.[23]
with the integral over the treatment time, R=0.5 for temperatures over 43 °C and 0.25 for temperatures between 43 °C and 37 °C, a reference temperature of 43 °C, and time T is in minutes. The equations and methods represent an approach for thermal dose estimation in an incompressible material such as tap water.[24]
An ultrasound acoustic wave cannot propagate through compressible tissue, such as rubber or human tissues. In that case the ultrasound energy is converted to heat. Using focused beams, a small region of heating can be achieved deep in tissues (usually on the order of 2~3 mm). Tissue changes as a function of the subtle shaking from the heated water within and the duration of this heating according to the thermal dose metric. Focusing at more than one place or by scanning, a volume can be ablated.[25][26][27] Thermal doses of 120-240 min at 43 °C coagulate cellular protein and lead to irreversible tissue destruction.
Cavitation
Inertial
At high enough acoustic intensities, cavitation (microbubbles forming and interacting with the ultrasound field) can occur. Microbubbles produced in the field oscillate and grow (due to factors including rectified diffusion), and can eventually implode (inertial or transient cavitation). During inertial cavitation, temperatures increase inside the bubbles. The ultimate collapse during the rarefaction phase is associated with a shock wave and jets that can mechanically damage tissue.[28]
Stable
Stable cavitation creates microstreaming, which induces high shear forces on cells and leads to apoptosis. Bubbles produced by the vaporization of water due to acoustic forces oscillate under a low-pressure acoustic field. Strong streaming may cause cell damage, but also reduces tissue temperature via convective heat loss.[29]
Theory
Ultrasound can be focused in several ways—via a lens (for example, a polystyrene lens, parabola curve transducer, or a phased array). This can be calculated using an exponential model of ultrasound attenuation. The ultrasound intensity profile is bounded by an exponentially decreasing function where the decrease in ultrasound is a function of distance traveled through tissue:
is the initial intensity of the beam, is the attenuation coefficient (in units of inverse length), and z is the distance traveled through the attenuating medium (e.g. tissue).
In this ideal model, [30] is a measure of the power density of the heat absorbed from the ultrasound field. This demonstrates that tissue heating is proportional to intensity, and that intensity is inversely proportional to the area over which an ultrasound beam is spread. Therefore, narrowly focusing the beam or increasing the beam intensity creates a rapid temperature rise at the focus.
The ultrasound beam can be focused in several ways:
- Geometrically, with a lens or with a spherically curved transducer.
- Electronically, by adjusting the relative phases of elements in an array of transducers (a "phased array"). This steers the beam to different locations. Aberrations in the ultrasound beam due to tissue structures can be corrected. This assumes no reflection, no absorption and no diffusion in intermediate tissue. The ultrasound itself can penetrate incompressible materials such as water, but compressible materials such as air, rubber, human tissue, fat, fiber, hollow bone, and fascia reflect, absorb, and diffuse the energy.
Beam delivery
Beam delivery consists of beam steering and image guidance. The beam has the ability to pass through overlying tissues without harm and focus on a localized area of 2–3 mm (at most), that determines the frequency of the ultrasound. Following ablation a distinct boundary (less than 50 microns wide) forms between healthy and necrotic tissue.[31]
Beam steering
The most common transducer is a concave focusing transducer with a fixed aperture and a fixed focal length.[31] Phased array transducers can be used with different arrangements (flat/bowl).[31]
Beam guidance
HIFU therapy requires careful monitoring and so it is usually performed in conjunction with other imaging techniques.
Pre-operative imaging, for instance CT and MRI, are used to identify general parameters of the target anatomy. Real-time imaging provides safe and accurate noninvasive targeting and monitoring. Both MRI and medical ultrasound have been used. These techniques are known respectively as Magnetic Resonance guided Focused Ultrasound Surgery (MRgFUS)[32][33] and Ultrasound guided Focused Ultrasound Surgery (USgFUS) respectively.[1][34]
MRgFUS is a 3D imaging technique. It features high soft tissue contrast and provides information about temperature, thus allowing ablation to be monitored. However, low frame rates make this technique perform poorly in real-time imaging while high costs limit its use.[35]
USgFUS is a 2D imaging technique. While no quantitative temperature measurement system is available, benefits such as high frame rate (up to 1000 images per second), low cost and minimal adverse health effects commend it. Ultrasound verifies the acoustic window in real time using the same modality as the therapy.[36] This implies that if the target region is not visualized by ultrasound imaging before and during therapy, then it is unlikely that the therapy will be effective in that specific region.[36] In addition, treatment outcomes can be estimated in real time through visual inspection of hyperechoic changes in standard B-mode images.[37]
References
- ^ a b c Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH (January 2008). "High-intensity focused ultrasound: current potential and oncologic applications". AJR. American Journal of Roentgenology. 190 (1): 191–199. doi:10.2214/AJR.07.2671. PMID 18094311.
- ^ Murat FJ, Poissonier L, Gelet A (2007). "Recurrent Prostate Cancer After Radiotherapy – Salvage Treatment by High-intensity Focused Ultrasound". European Oncological Disease. 1 (1): 60–2. Archived from the original on 4 October 2013. Retrieved 4 October 2013.
- ^ "Food and Drug Administration Approval, ExAblate® 2000 System – P040003". Food and Drug Administration. Archived from the original on 9 July 2009. Retrieved 2 December 2023.
- ^ FDA News Release. "FDA approves first MRI-guided focused ultrasound device to treat essential tremor", FDA, July 11, 2016
- ^ Martin-Fiori, E (2014). "Functional Neurosurgery with MR-Guided HIFU". Intraoperative Imaging and Image-Guided Therapy. New York: Springer. pp. 591–599. doi:10.1007/978-1-4614-7657-3_45. ISBN 978-1-4614-7657-3.
- ^ "Histotripsy Group". Histotripsy Group. Retrieved 3 March 2025.
- ^ Robertson VJ, Baker KG (July 2001). "A review of therapeutic ultrasound: effectiveness studies". Physical Therapy. 81 (7): 1339–1350. doi:10.1093/ptj/81.7.1339. PMID 11444997.
- ^ Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. (August 2013). "A pilot study of focused ultrasound thalamotomy for essential tremor". The New England Journal of Medicine. 369 (7): 640–648. doi:10.1056/NEJMoa1300962. PMID 23944301.
- ^ Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. (January 2012). "Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain". Neurosurgical Focus. 32 (1): E1. doi:10.3171/2011.10.FOCUS11248. PMID 22208894. S2CID 2231685.
- ^ Magara A, Bühler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D (2014). "First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease". Journal of Therapeutic Ultrasound. 2 11. doi:10.1186/2050-5736-2-11. PMC 4266014. PMID 25512869.
- ^ Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C (2011). "More than just tumor destruction: immunomodulation by thermal ablation of cancer". Clinical & Developmental Immunology. 2011: 160250. doi:10.1155/2011/160250. PMC 3254009. PMID 22242035.
- ^ Wu F (August 2013). "High intensity focused ultrasound ablation and antitumor immune response". The Journal of the Acoustical Society of America. 134 (2): 1695–1701. Bibcode:2013ASAJ..134.1695W. doi:10.1121/1.4812893. PMID 23927210.
- ^ Chaussy CG, Thüroff S (April 2017). "High-Intensity Focused Ultrasound for the Treatment of Prostate Cancer: A Review". Journal of Endourology. 31 (S1): S30 – S37. doi:10.1089/end.2016.0548. PMID 28355119.
- ^ Hu JC, Laviana A, Sedrakyan A (June 2016). "High-Intensity Focused Ultrasound for Prostate Cancer: Novelty or Innovation?". JAMA. 315 (24): 2659–2660. doi:10.1001/jama.2016.5002. PMID 27367874.
- ^ Lepor H, Gold S, Wysock J (2018). "Focal Ablation of Prostate Cancer". Reviews in Urology. 20 (4): 145–157. doi:10.3909/riu0809 (inactive 1 July 2025). PMC 6375006. PMID 30787673.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ Ng KK, Poon RT, Chan SC, Chok KS, Cheung TT, Tung H, et al. (May 2011). "High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience". Annals of Surgery. 253 (5): 981–987. doi:10.1097/SLA.0b013e3182128a8b. hdl:10722/135541. PMID 21394012. S2CID 25603451.
- ^ Mauri G, Nicosia L, Xu Z, Di Pietro S, Monfardini L, Bonomo G, et al. (February 2018). "Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer". The British Journal of Radiology. 91 (1083): 20170641. doi:10.1259/bjr.20170641. PMC 5965486. PMID 29168922.
- ^ Mendiratta-Lala M, Wiggermann P, Pech M, Serres-Créixams X, White SB, Davis C, et al. (September 2024). "The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors". Radiology. 312 (3) e233051. doi:10.1148/radiol.233051. PMC 11427859. PMID 39225612.
- ^ "Evidence". HistoSonics. Retrieved 3 March 2025.
- ^ Sandilos G, Butchy MV, Koneru M, Gongalla S, Sensenig R, Hong YK (August 2024). "Histotripsy - hype or hope? Review of innovation and future implications". Journal of Gastrointestinal Surgery. 28 (8): 1370–1375. doi:10.1016/j.gassur.2024.05.038. PMID 38862075.
- ^ Xu Z, Hall TL, Vlaisavljevich E, Lee FT (1 January 2021). "Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound". International Journal of Hyperthermia. 38 (1): 561–575. doi:10.1080/02656736.2021.1905189. PMC 9404673. PMID 33827375.
- ^ Zhou YF (January 2011). "High intensity focused ultrasound in clinical tumor ablation". World Journal of Clinical Oncology. 2 (1): 8–27. doi:10.5306/wjco.v2.i1.8. PMC 3095464. PMID 21603311.
- ^ Sapareto SA, Dewey WC (June 1984). "Thermal dose determination in cancer therapy". International Journal of Radiation Oncology, Biology, Physics. 10 (6): 787–800. doi:10.1016/0360-3016(84)90379-1. PMID 6547421.
- ^ Mouratidis PX, Rivens I, Civale J, Symonds-Tayler R, Ter Haar G (1 January 2019). "'Relationship between thermal dose and cell death for "rapid" ablative and "slow" hyperthermic heating'". International Journal of Hyperthermia. 36 (1): 229–243. doi:10.1080/02656736.2018.1558289. PMID 30700171.
- ^ Huisman M, Lam MK, Bartels LW, Nijenhuis RJ, Moonen CT, Knuttel FM, et al. (2014). "Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases". Journal of Therapeutic Ultrasound. 2: 16. doi:10.1186/2050-5736-2-16. PMC 4193684. PMID 25309743.
- ^ Köhler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. (August 2009). "Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry". Medical Physics. 36 (8): 3521–3535. Bibcode:2009MedPh..36.3521K. doi:10.1118/1.3152112. PMID 19746786.
- ^ Monteith SJ, Kassell NF, Goren O, Harnof S (May 2013). "Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage". Neurosurgical Focus. 34 (5): E14. doi:10.3171/2013.2.FOCUS1313. PMID 23634918.
- ^ Leighton TG (1997). "Chapter 9: The principles of cavitation". Ultrasound in food processing. Thomson Science, London, Blackie Academic and Professional. pp. 151–182.
- ^ Levario-Diaz V, Bhaskar P, Carmen Galan M, Barnes AC (May 2020). "Effect of acoustic standing waves on cellular viability and metabolic activity". Scientific Reports. 10 (1) 8493. Bibcode:2020NatSR..10.8493L. doi:10.1038/s41598-020-65241-4. PMC 7244593. PMID 32444830.
- ^ Hariharan P, Myers MR, Banerjee RK (June 2007). "HIFU procedures at moderate intensities--effect of large blood vessels". Physics in Medicine and Biology. 52 (12): 3493–3513. Bibcode:2007PMB....52.3493H. doi:10.1088/0031-9155/52/12/011. PMID 17664556. S2CID 26124121.
- ^ a b c Izadifar Z, Izadifar Z, Chapman D, Babyn P (February 2020). "An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications". Journal of Clinical Medicine. 9 (2): 460. doi:10.3390/jcm9020460. PMC 7073974. PMID 32046072.
- ^ Kotopoulis S, Wang H, Cochran S, Postema M (August 2011). "Lithium niobate transducers for MRI-guided ultrasonic microsurgery" (PDF). IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 58 (8): 1570–1576. Bibcode:2011ITUFF..58.1570K. doi:10.1109/TUFFC.2011.1984. PMID 21859576. S2CID 11382728.
- ^ Medel R, Monteith SJ, Elias WJ, Eames M, Snell J, Sheehan JP, et al. (October 2012). "Magnetic resonance-guided focused ultrasound surgery: Part 2: A review of current and future applications". Neurosurgery. 71 (4): 755–763. doi:10.1227/NEU.0b013e3182672ac9. PMC 4104674. PMID 22791029.
- ^ Belzberg M, Mahapatra S, Perdomo-Pantoja A, Chavez F, Morrison K, Xiong KT, et al. (December 2020). "Minimally invasive therapeutic ultrasound: Ultrasound-guided ultrasound ablation in neuro-oncology". Ultrasonics. 108 (12) 106210. doi:10.1016/j.ultras.2020.106210. PMC 8895244. PMID 32619834.
- ^ Cafarelli A, Mura M, Diodato A, Schiappacasse A, Santoro M, Ciuti G, et al. (25–29 August 2015). "A computer-assisted robotic platform for Focused Ultrasound Surgery: Assessment of high intensity focused ultrasound delivery". 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). pp. 1311–1314. doi:10.1109/EMBC.2015.7318609. ISBN 978-1-4244-9271-8. PMID 26736509. S2CID 4194253.
- ^ a b Chen PH, Hsieh KS, Huang CC (2017). "An Acoustic Tracking Approach for Medical Ultrasound Image Simulator". Journal of Medical and Biological Engineering. 37 (6): 944–952. doi:10.1007/s40846-017-0258-9. PMC 6208925. PMID 30416414.
- ^ Ebbini ES, ter Haar G (March 2015). "Ultrasound-guided therapeutic focused ultrasound: current status and future directions". International Journal of Hyperthermia. 31 (2): 77–89. doi:10.3109/02656736.2014.995238. PMID 25614047. S2CID 23590340.