Flumioxazin
 | |
| Names | |
|---|---|
| IUPAC name
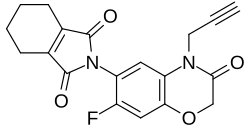
2-[7-fluoro-3-oxo-4-(prop-2-ynyl)-2H,4H-1,4-benzoxazin-6-yl]-4,5,6,7-tetrahydro-1H-isoindole-1,3(2H)-dione
| |
| Other names
Flumioxazine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.113.142 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H15FN2O4 | |
| Molar mass | 354.337 g·mol−1 |
| Hazards | |
| GHS labelling:[1] | |
| |
| Warning | |
| H361d, H410 | |
| P203, P273, P280, P318, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Flumioxazin is a synthetic herbicide used for control of broadleaf weeds in agricultural areas.[1] Valent U.S.A. Corporation, a division of Sumitomo Chemical, developed flumioxazin, which was approved by the U.S. EPA in 2001 for use on soybean and peanut crops.[2] Flumioxazin has gained popularity due to pesticide resistance toward earlier active ingredients.[3]
Flumioxazin is also used to control aquatic plants such as filamentous algae. In granular form, it is used to control of submerged plants, and as a direct foliar application it is used to control emergent and floating-leaf plants.[4]
Mode of Action
Flumioxazin is an inhibitor of the enzyme protoporphyrinogen oxidase which then interferes with the plant's chlorophyll production.[5]
Flumioxazin's HRAC classification is Group G (Australia), Group E (global), or Group 14 (numeric).[6]
Manufacture
The production of flumioxazin involves the use of 2,4-difluoronitrobenzene as a crucial raw material.[3]
References
- ^ "Flumioxazin: Environmental Fate and Ecological Risk Assessment" (PDF). United States Environmental Protection Agency.
- ^ "Flumioxazin: Pesticide Fact Sheet" (PDF). U.S. EPA. 2001. Retrieved 2 May 2025.
- ^ a b Guo, Shuai; Zhan, Le-wu; Li, Bin-dong (December 2023). "Mixing intensification and kinetics of 2,4-difluoronitrobenzene homogeneous nitration reaction in a heart-shaped continuous-flow microreactor". Chemical Engineering Journal. 477 147011. Bibcode:2023ChEnJ.47747011G. doi:10.1016/j.cej.2023.147011.
- ^ "Flumioxazin Chemical Fact Sheet" (PDF). Wisconsin Department of Natural Resources. 2012.
- ^ Iwashita, Katsumasa; Hosokawa, Yoshinori; Ihara, Ryo; Miyamoto, Taiki; Otani, Mitsuhiro; Abe, Jun; Asano, Koji; Mercier, Odile; Miyata, Kaori; Barlow, Susan (2022). "Flumioxazin, a PPO inhibitor: A weight-of-evidence consideration of its mode of action as a developmental toxicant in the rat and its relevance to humans". Toxicology. 472 153160. Bibcode:2022Toxgy.47253160I. doi:10.1016/j.tox.2022.153160. PMID 35367320.
- ^ "Classification of Herbicides According to Site of Action". Retrieved 19 July 2025.