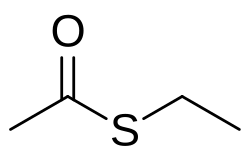
Ethyl thioacetate
 | |
| Names | |
|---|---|
| Other names
S-ethyl thioacetate, S-ethyl thiolacetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.009.914 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8OS | |
| Molar mass | 104.17 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.971 g/cm³ |
| insoluble | |
| Solubility | diethyl ether, ethanol |
Refractive index (nD)
|
1.456-1.468 |
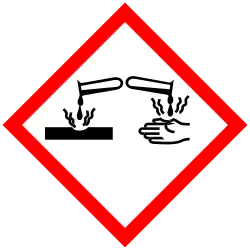
| Hazards | |
| GHS labelling:[1] | |
| |
| Warning | |
| H225, H302, H315, H318, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P303+P361+P353, P304+P340, P305+P354+P338, P317, P319, P321, P330, P332+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Ethyl thioacetate is the organosulfur compound with the formula CH3C(O)SC2H5. It is an ethyl ester of thioacetic acid. The compound is traditionally prepared by alkylation of potassium thioacetate and thiolation of acetyl chloride:[1]
- CH3COSK + C2H5Br → CH3C(O)SC2H5 + KBr
- CH3COCl + C2H5SH → CH3C(O)SC2H5 + HCl
The compound has been well studied in part as a model for biologically relevant thioesters, such as Coenzyme A. H-D exchange of the methyl group has been examined in aqueous solution.[2]
Related compounds
- [[O-Ethyl thioacetate]|O-Ethyl thioacetate]] (C2H5OC(S)CH3) is the unstable isomer of S-ethyl thioacetate
References
- ^ Matthys J. Janssen (1969). "Thiolo, Thiono and Dithio Acids and Esters". In Saul Patai (ed.). Carboxylic Acids and Esters. PATAI's Chemistry of Functional Groups. New York: John Wiley. pp. 705–764. doi:10.1002/9780470771099.ch15. ISBN 978-0-471-66919-7.
- ^ Amyes, Tina L.; Richard, John P. (1992). "Generation and stability of a simple thiol ester enolate in aqueous solution". Journal of the American Chemical Society. 114 (26): 10297–10302. Bibcode:1992JAChS.11410297A. doi:10.1021/ja00052a028.