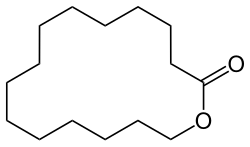
Cyclopentadecanolide
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Oxacyclohexadecan-2-one | |
| Other names
Angelica lactone; Muskalactone; Muskolactone; Exaltolide; Pentalide; Pentadecanolide; Pentadecalactone; 15-Hydroxypentadecanoic acid, lactone; 15-Hydroxypentadecanoic acid-epsilon-lactone; Pentadecanoic acid, 15-hydroxy-, E-lactone; ω-Pentadecalactone; omega-Pentadecalactone; ω-Lactone; 2-Pentadecalone; Pentadecan-15-olide; 1,15-Pentadecanolide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.050 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H28O2 | |
| Molar mass | 240.387 g·mol−1 |
| Appearance | Colorless crystals |
| Odor | Musklike |
| Density | 0.940 |
| Melting point | 34 °C (93 °F; 307 K)[1] |
| Boiling point | 98 °C (208 °F; 371 K)[2] at 0.02 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Cyclopentadecanolide is an organic compound with the formula (CH2)11CO2. A colorless solid, it is classified as macrolide lactone. It has some use in perfumery.
Occurrence and production
Cyclopentadecanolide occurs in small quantities in angelica root essential oil and is responsible for its musklike odor.[3]
Cyclopentadecanolide is produced by ring expansion of cyclotetradecanone. Another synthesis route is the depolymerization of polyesters of 15-hydroxypentadecanoic acid.[3]
Uses
Cyclopentadecanolide is used as a musklike perfume fixative in fine fragrances and as a flavoring agent.[4] It is a substitute for the extremely expensive animal musk.[3]
References
- ^ Morales-Serna, José Antonio; Sánchez, Ericka; Velázquez, Ricardo; Bernal, Jorge; García-Ríos, Eréndira; Gaviño, Rubén; Negrón-Silva, Guillermo; Cárdenas, Jorge (2010). "Highly efficient macrolactonization of ω-hydroxy acids using benzotriazole esters: synthesis of Sansalvamide A". Organic & Biomolecular Chemistry. 8 (21): 4940–4948. doi:10.1039/c0ob00161a. ISSN 1477-0520. PMID 20820651.
- ^ Bestmann, Hans Jürgen; Schobert, Rainer (1989). "Kumulierte Ylide XX. Synthesen (E)-α,β-ungesättigter macrocyclischer Lactone durch intramolekulare Wittig-Olefinierung via Triphenylphosphoranylidenkete2". Synthesis (in German). 1989 (6): 419–423. doi:10.1055/s-1989-27271. ISSN 0039-7881. Archived from the original on 2018-06-05. Retrieved 2021-12-06.
- ^ a b c Fahlbusch, Karl-Georg; et al. (2007). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry (7th ed.). Wiley. p. 75.
- ^ Burdock, George A. (2010). "ω-PENTADECALACTONE". Fenaroli's Handbook of Flavor Ingredients (6th ed.). Taylor & Francis. p. 1597. ISBN 9781420090772.