Cipargamin
 | |
| Names | |
|---|---|
| Systematic IUPAC name
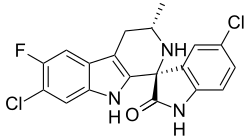
(1′R,3′S)-5,7′-Dichloro-6′-fluoro-3′-methyl-2′,3′,4′,9′-tetrahydrospiro[indole-3,1′-pyrido[3,4-b]indol]-2(1H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H14Cl2FN3O | |
| Molar mass | 390.24 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Cipargamin (also known as KAE609 or NITD609) is a synthetic antimalarial compound belonging to the novel spiroindolone drug class.[1] Developed by Novartis Institute for Tropical Diseases in Singapore, through a collaboration with the Genomics Institute of the Novartis Research Foundation (GNF), the Biomedical Primate Research Centre and the Swiss Tropical Institute, cipargamin represents a promising next-generation antimalarial drug currently undergoing Phase II clinical trials with a particular focus on safety evaluation.[1][2][3] Cipargamin was awarded MMV Project of the Year 2009[4].
Mechanism of action
Cipargamin exerts its antimalarial activity by targeting PfATP4, a P-type Na+ ATPase located on the plasma membrane of Plasmodium parasites.[1][5] By inhibiting this essential sodium pump, cipargamin disrupts the sodium homeostasis within the parasite, leading to cell swelling and ultimately parasite death.[1][6] This mechanism of action distinguishes cipargamin from existing antimalarial drugs, including artemisinin derivatives and other peroxide-based compounds.
Structure-activity relationships
The spiroindolone structure of cipargamin features a complex stereochemical configuration that is critical for its antimalarial activity.[7] Structure-activity relationship studies have demonstrated that the (1R,3S) stereochemical configuration is essential for optimal antimalarial potency.[8] It is structurally related to GNF 493, a compound first identified as a potent inhibitor of Plasmodium falciparum growth in a high throughput phenotypic screen of natural products conducted at the Genomics Institute of the Novartis Research Foundation in San Diego, California in 2006.[4]
Clinical development
Phase I studies
Initial Phase I clinical trials evaluated the safety, tolerability, and pharmacokinetics of cipargamin in healthy volunteers and patients with uncomplicated P. falciparum malaria. These studies demonstrated rapid parasite clearance and established preliminary dose ranges for subsequent Phase II investigations.[9]
Phase II studies
A randomized, dose-escalation Phase II study conducted in Sub-Saharan Africa evaluated single oral doses of cipargamin ranging from 30 to 150 mg in adults with uncomplicated P. falciparum malaria.[10] The study demonstrated that cipargamin at doses of 50-150 mg was associated with very rapid parasite clearance and achieved PCR-corrected adequate clinical and parasitological response rates exceeding 65% at 28 days.[10] A separate Phase II study in Thailand evaluated a 3-day regimen of 30 mg cipargamin daily, which successfully cleared parasitemia in patients infected with both P. falciparum and P. vivax.[11]
Intravenous formulation development
Current clinical development includes evaluation of an intravenous formulation of cipargamin for the treatment of severe malaria, representing a potential alternative to injectable artesunate.[12] This development is particularly significant given the emerging threat of artemisinin resistance in Asian countries and the need for alternative treatments for severe malaria, which primarily affects young children in Africa.[12]
Adverse events
Clinical studies have demonstrated that cipargamin is generally well-tolerated, with adverse events similar across different dose groups.[10] The most commonly reported adverse events were malaria-related symptoms including headache, malaria recurrence/re-infection, and other treatment-related symptoms.[10] Importantly, Phase II trials have included specific focus on hepatic safety monitoring, with one isolated case of transient ALT elevation that normalized by day 8 following treatment.[10]
Resistance mechanisms
Research has identified specific resistance mechanisms to cipargamin, primarily involving mutations in the pfatp4 gene encoding the target P-type ATPase.[13] A clinically relevant G358S mutation in PfATP4 has been identified as conferring significant resistance to cipargamin treatment.[14] Clinical studies have documented that recrudescent parasites frequently harbor treatment-emerging mutations, indicating the potential for resistance development under selective pressure.[10]
History
Cipargamin was discovered by screening the Novartis library of 12,000 natural products and synthetic compounds to find compounds active against Plasmodium falciparum. The first screen turned up 275 compounds and the list was narrowed to 17 potential candidates. The current spiroindolone was optimized to address its metabolic liabilities leading to improved stability and exposure levels in animals. As a result, cipargamin is one of only a handful of molecules capable of completely curing mice infected with Plasmodium berghei (a model of blood-stage malaria).
References
- ^ a b c d Stein, Dörte S.; Jullian, Natacha; Docampo, Roberto; Ramirez, Jose Luis; Winzeler, Elizabeth A.; Wittlin, Sergio; Charman, Susan A.; Creek, Darren J. (2020). "The early preclinical and clinical development of cipargamin (KAE609), a novel antimalarial compound". Travel Medicine and Infectious Disease. 36: 101629. doi:10.1016/j.tmaid.2020.101629. PMC 7206223. PMID 32179122.
- ^ "NITD 609". Medicines for Malaria Venture. Archived from the original on 12 November 2012. Retrieved 17 April 2013.
- ^ Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT (2010). "Spiroindolones, a potent compound class for the treatment of malaria". Science. 329 (5996): 1175–80. Bibcode:2010Sci...329.1175R. doi:10.1126/science.1193225. PMC 3050001. PMID 20813948.
- ^ a b "MMV's Project of the Year for 2009 goes to Spiroindolones". Medicines for Malaria Venture. 15 March 2010. Retrieved 25 July 2025.
- ^ Spillman, Natalie J.; Allen, Rebecca J. W.; McNamara, Case W.; Yeung, Brian K. S.; Winzeler, Elizabeth A.; Diagana, Thierry T.; Kirk, Kiaran (2013). "Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4". Journal of Biological Chemistry. 288: 9418–9426. doi:10.1074/jbc.M112.442168 (inactive 25 July 2025).
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ Spillman, Natalie J.; Kirk, Kiaran (2018). "Cell Swelling Induced by the Antimalarial KAE609 (Cipargamin) and Other PfATP4-Associated Antimalarials". Antimicrobial Agents and Chemotherapy. 62 (6). doi:10.1128/AAC.00087-18. PMC 5971608. PMID 29555632.
- ^ Yeung, Brian K. S.; Zou, Bin; Rottmann, Matthias; Lakshminarayana, Suresh B.; Ang, Sarah H.; Chia, Sharon Y.; Diagana, Thierry T. (2010). "Spirotetrahydro β-carbolines (spiroindolones): a new class of potent and orally active antimalarials". Journal of Medicinal Chemistry. 53 (14): 5155–5164. doi:10.1021/jm100410f. PMC 6996867. PMID 20568778.
- ^ White, Karen L.; Shackleford, David M.; Shackleford, Giang; Montangero, Daniel; Charman, Susan A. (2013). "Optimization of the spirocyclic core of spiroindolone antimalarials". ACS Medicinal Chemistry Letters. 4 (12): 1158–1162. doi:10.1021/ml400296k (inactive 25 July 2025).
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ Phyo, Aung Pyae; Jittamala, Podjanee; Nosten, François H.; Pukrittayakamee, Sasithon; Imwong, Mallika; White, Nicholas J.; Duparc, Stephan; Macintyre, Fiona; Baker, Mark; Möhrle, Jörg J. (1 January 2016). "Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial". The Lancet. Infectious Diseases. 16 (1): 61–69. doi:10.1016/S1473-3099(15)00320-5. ISSN 1474-4457. PMC 4700386. PMID 26448141.
- ^ a b c d e f White, Nicholas J.; Pukrittayakamee, Sasithon; Phyo, Aung Pyae; Rueangweerayut, Ronnatrai; Nosten, François; Jittamala, Podjanee; Charman, Susan A. (2022). "Efficacy of Cipargamin (KAE609) in a Randomized, Phase II Dose-Escalation Study in Adults in Sub-Saharan Africa With Uncomplicated Plasmodium falciparum Malaria". Clinical Infectious Diseases. 74 (10): 1831–1839. doi:10.1093/cid/ciab717.
- ^ Creek, Darren J.; Charman, Susan A.; Gilbert, Ian H.; Huston, Charles D.; Kappe, Stefan H. I.; Kyle, Dennis E. (2021). "Defining the Antimalarial Activity of Cipargamin in Healthy Volunteers Experimentally Infected with Blood-Stage Plasmodium falciparum". Antimicrobial Agents and Chemotherapy. 65 (2). doi:10.1128/AAC.01423-20. PMC 7849011. PMID 33199389.
- ^ a b "To Evaluate Efficacy, Safety, Tolerability and PK of Intravenous Cipargamin in Participants With Severe Plasmodium Falciparum Malaria". Clinical Trials.gov. 2023. Retrieved 25 July 2025.
- ^ Jiménez-Díaz, María Belén; Ebert, Diane; Salinas, Yolanda; Pradhan, Aneesh; Lehane, Adele M.; Myrand-Lapierre, Marie-Eve; Kirk, Kiaran (2014). "(+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium". Proceedings of the National Academy of Sciences. 111 (50): E5455 – E5462. Bibcode:2014PNAS..111E5455J. doi:10.1073/pnas.1414221111. PMC 4273362. PMID 25453091.
- ^ Spillman, Natalie J.; Beck, Jennifer R.; Ganesan, Sachel M.; Niles, Jacquin C.; Goldberg, Daniel E.; Fidock, David A.; Kirk, Kiaran (2022). "A G358S mutation in the Plasmodium falciparum Na+ pump PfATP4 confers clinically-relevant resistance to cipargamin". Nature Communications. 13 (1) 5746. doi:10.1038/s41467-022-33403-9. PMC 9525273. PMID 36180431.