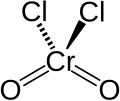
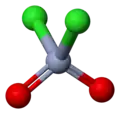
Chromyl chloride
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chromium(VI) dichloride dioxide | |||
| Systematic IUPAC name
Dichloridodioxidochromium | |||
| Other names
Chromic acid chloride
Chromium oxychloride Chromic oxychloride Étard reagent Chlorochromic anhydride Chromium chloride oxide Chromium dioxide dichloride Chromium dioxychloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.035.491 | ||
| EC Number |
| ||
| 2231 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1758 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CrO2Cl2 | |||
| Molar mass | 154.9008 g/mol | ||
| Appearance | Blood-red fuming liquid, similar to bromine | ||
| Odor | Musty, burning, acrid[1] | ||
| Density | 1.911 g/mL, liquid | ||
| Melting point | −96.5 °C (−141.7 °F; 176.7 K) | ||
| Boiling point | 118.5 °C (245.3 °F; 391.6 K) | ||
| Reacts with water | |||
| Vapor pressure | 20 mmHg (20 °C)[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, oxidizer, carcinogenic, mutagenic, reacts violently with water[1] | ||
| GHS labelling: | |||
| |||
| Danger | |||
| H271, H314, H317, H340, H350, H410 | |||
| P201, P210, P280, P303+P361+P353, P305+P351+P338+P310, P308+P313[2] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | noncombustible[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
Ca TWA 0.001 mg Cr(VI)/m3[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
| Safety data sheet (SDS) | Sigma Aldrich - Chromyl Chloride | ||
| Related compounds | |||
Related compounds
|
Sulfuryl chloride Vanadium oxytrichloride Molybdenum dichloride dioxide Tungsten dichloride dioxide Chromyl fluoride Uranyl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds.[3]
Preparation
Chromyl chloride can be prepared by the reaction of potassium chromate or potassium dichromate with hydrogen chloride in the presence of concentrated sulfuric acid, followed by distillation.[4][5]
- K2Cr2O7 + 6 HCl → 2 CrO2Cl2 + 2 KCl + 3 H2O
The sulfuric acid serves as a dehydration agent. This reaction, the chloride test, gives a strikingly red product. It was once used as a test for chloride. No similar compound is formed in the presence of fluoride, bromide, iodide, or cyanide, making this test specific to chlorides.
It can also be prepared directly by exposing chromium trioxide to anhydrous hydrogen chloride gas.
- CrO3 + 2 HCl ⇌ CrO2Cl2 + H2O
When trityl chloride is used in place of HCl, the products are CrO2Cl2 and the alkoxides CrO2Cl(OCPh3) and CrO2(OCPh3)2 (Ph = C6H5).[6]
Reactions
Chromyl chloride is a Lewis acid, and a highly aggressive oxidant. It reacts with cyclohexane to give chlorocyclohexane, chlorocyclohexanone, and other species.[7]
Chromyl chloride oxidizes internal alkenes to alpha-chloroketones or related derivatives.[8] It will also attack benzylic methyl groups to give aldehydes via the Étard reaction. Dichloromethane is a suitable solvent for these reactions.[9]
When treated with conventional Lewis bases, chromyl chloride forms adducts, such as CrO2Cl2(2,2'-bipy):[10]
- CrO2Cl2 + 2 L → CrO2Cl2L2
Safety considerations

Chromyl chloride is severely corrosive and easily burns the skin and eyes. It is a probable human carcinogen.[11]
References
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0142". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Chromyl chloride 200042" (PDF). Sigma-Aldrich. Archived from the original on 2020-09-01.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1022. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Moody, B.J. (1965). "22". Comparative Inorganic Chemistry (1 ed.). London: Edward Arnold. p. 381. ISBN 0-7131-3679-0.
- ^ Sisler, Harry H. (1946). "Chromyl Chloride [Chromium(VI) Dioxychloride]". Inorganic Syntheses. Vol. 2. pp. 205–207. doi:10.1002/9780470132333.ch63. ISBN 9780470132333.
{{cite book}}: ISBN / Date incompatibility (help) - ^ Stavropoulos, Pericles; Bryson, Nathan; Youinou, Marie Therese; Osborn, John A. (1990). "Chromyl Complexes with Aryloxy and Siloxy Ligands". Inorganic Chemistry. 29 (10): 1807–1811. doi:10.1021/ic00335a009.
- ^ Gunay, Ahmet; Theopold, Klaus H. (2010). "C−H Bond Activations by Metal Oxo Compounds". Chemical Reviews. 110 (2): 1060–1081. doi:10.1021/cr900269x. PMID 20143877.
- ^ Freeman, Fillmore; DuBois, Richard H.; McLaughlin, Thomas G. (1971). "Aldehydes by Oxidation of Terminal Olefins with Chromyl Chloride: 2,4,4-Trimethylpentanal". Org. Synth. 51: 4. doi:10.15227/orgsyn.051.0004.
- ^ F. Freeman (2004). "Chromyl Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc177. ISBN 0471936235.
- ^ Levason, William; Reid, Gillian; Zhang, Wenjian (2014). "Synthesis, Properties, and Structures of Chromium(VI) and Chromium(V) Complexes with Heterocyclic Nitrogen Ligands". Zeitschrift für Anorganische und Allgemeine Chemie. 640 (1): 35–39. Bibcode:2014ZAACh.640...35L. doi:10.1002/zaac.201300482.
- ^ Prof CH Gray, ed. (1966). "IV". Laboratory Handbook of Toxic Agents (2 ed.). London: Royal Institute of Chemistry. p. 79.