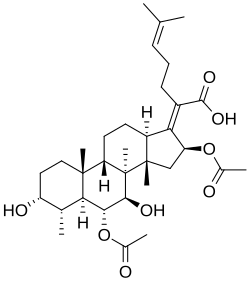
Cephalosporin P1
 | |
| Names | |
|---|---|
| IUPAC name
(2Z)-2-[(3R,4S,5S,6R,7R,8S,9S,10R,13R,14S,16S)-6,16-diacetyloxy-3,7-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic acid
| |
| Other names
Acremonic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C33H50O8 | |
| Molar mass | 574.755 g·mol−1 |
| Density | g/cm³ |
| poorly soluble[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Cephalosporin P1 is a naturally occurring antibiotic compound belonging to the cephalosporin class, which was first isolated from the fungus Cephalosporium acremonium (later reclassified as Acremonium chrysogenum)[2]. It is one of the early cephalosporins discovered, alongside cephalosporin C and other related metabolites.
Fusidane-type antibiotics are a class of triterpenoid antibiotics that include helvolic acid, fusidic acid, and cephalosporin P1.[3] Among these, fusidic acid is notable for its clinical use in treating bacterial infections.[4]
Uses
Unlike later-generation cephalosporins used clinically, cephalosporin P1 has limited therapeutic use due to its weaker antibacterial activity.
Cephalosporin P1 demonstrated strong effectiveness against methicillin-sensitive Staphylococcus aureus, methicillin-resistant S. aureus, and vancomycin-intermediate S. aureus strains.[5]
References
- ^ Bycroft, Barrie W.; Payne, David J. (9 August 2013). Dictionary of Antibiotics and Related Substances: Second Edition. CRC Press. p. 463. ISBN 978-1-4822-8215-3. Retrieved 24 July 2025.
- ^ Chou, T. S.; Eisenbraun, E. J.; Rapala, R. T. (1 January 1967). "The chemistry of cephalosporin P1". Tetrahedron Letters. 8 (5): 409–414. doi:10.1016/S0040-4039(00)90960-2. ISSN 0040-4039. Retrieved 24 July 2025.
- ^ Advances in Applied Microbiology. Academic Press. 18 January 1980. p. 99. ISBN 978-0-08-056439-5. Retrieved 24 July 2025.
- ^ Cao, Zhi-Qin; Lv, Jian-Ming; Liu, Qiu; Qin, Sheng-Ying; Chen, Guo-Dong; Dai, Ping; Zhong, Yue; Gao, Hao; Yao, Xin-Sheng; Hu, Dan (17 January 2020). "Biosynthetic Study of Cephalosporin P1 Reveals a Multifunctional P450 Enzyme and a Site-Selective Acetyltransferase". ACS Chemical Biology. 15 (1): 44–51. doi:10.1021/acschembio.9b00863. ISSN 1554-8929. Retrieved 24 July 2025.
- ^ O'Neill, A. J.; Bostock, J. M.; Moita, A. Morais; Chopra, I. (December 2002). "Antimicrobial activity and mechanisms of resistance to cephalosporin P1, an antibiotic related to fusidic acid". The Journal of Antimicrobial Chemotherapy. 50 (6): 839–848. doi:10.1093/jac/dkf248. ISSN 0305-7453. Retrieved 24 July 2025.