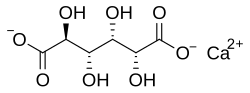
Calcium D-glucarate
 | |
| Names | |
|---|---|
| Other names
Calcium d-saccharate, Antacidin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.024.850 |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8CaO8 | |
| Molar mass | 248.200 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Calcium D-glucarate (or calcium saccharate) is the calcium salt of D-glucaric acid (saccharic acid), a substance naturally found in small amounts in fruits and vegetables such as oranges, apples, grapefruits, and cruciferous vegetables.[1][2] It is commonly used as a dietary supplement, primarily marketed for its potential role in supporting detoxification processes and hormone regulation.[2][3] Preclinical studies suggest that calcium D-glucarate may possess anticarcinogenic properties and improve detoxification markers, but more definitive clinical research such as a randomized controlled trial is needed to verify health claims.[4][5]
Structure and chemistry
Calcium D-glucarate is composed of calcium bound to D-glucaric acid, a derivative of glucose metabolism.[6] D-Glucaric acid itself is classified as a sugar acid, and when bonded with calcium, it forms a more stable compound suitable for supplementation.
Biological activity
In the body, calcium D-glucarate is hydrolyzed to release D-glucaric acid, which can be further metabolized into compounds such as D-glucaro-1,4-lactone.[7] These metabolites are known to inhibit the enzyme beta-glucuronidase.[7][8] Beta-glucuronidase is involved in the breakdown of glucuronides in the liver and intestines, a process that can release potentially harmful compounds back into circulation.[8]
By inhibiting beta-glucuronidase, calcium D-glucarate is thought to promote the excretion of carcinogens, steroid hormones (such as estrogen), and other potentially toxic substances bound for elimination through the bile and urine.[7][9]
Simulations suggest other mechanisms of detoxification such as inhibiting the production of reactive oxygen species, lowering deglucuronidation activity which would otherwise prevent the elimination of toxic substances from the body, or suppressing hepatocyte apoptosis. However, in vivo research is needed to verify the occurrence of such mechanistic pathways in humans.[5]
Dietary sources
Although calcium D-glucarate itself is not abundant in foods, D-glucaric acid is naturally present in fruits and vegetables.[1][2] Foods with notable amounts include:
- Oranges
- Apples
- Grapefruit
- Broccoli
- Brussels sprouts
Supplement use
Calcium D-glucarate is available as an over-the-counter dietary supplement, typically in capsule or tablet form. Dosages used in supplements generally range from 200 mg to 1000 mg per serving.[2]
Safety and side effects
Calcium D-glucarate appears to be well tolerated in humans when used at typical supplemental doses.[9][10] Reported side effects are rare but may include mild gastrointestinal discomfort. No major toxicity concerns have been identified in available studies, although long-term human research is limited[9][10]
See also
References
- ^ a b Vieths, S.; Schöning, B.; Jankiewicz, A. (January 1993). "Occurrence of IgE binding allergens during ripening of apple fruits". Food and Agricultural Immunology. 5 (2): 93–105. doi:10.1080/09540109309354788. ISSN 0954-0105.
- ^ a b c d Schattner, Mark A; Willis, Holly J; Raykher, Alexandra; Brown, Patricia; Quesada, Ofelia; Scott, Burma; Shike, Moshe (2006). "Long-term Enteral Nutrition Facilitates Optimization of Body Weight". Oncology & Hematology Review (2): 104. doi:10.17925/ohr.2006.00.02.104. ISSN 2052-3815.
- ^ Ershow, Abby; Skeaff, Sheila; Merkel, Joyce; Pehrsson, Pamela (2018-01-17). "Development of Databases on Iodine in Foods and Dietary Supplements". Nutrients. 10 (1): 100. doi:10.3390/nu10010100. ISSN 2072-6643. PMC 5793328. PMID 29342090.
- ^ Memorial Sloan Kettering Cancer Center (22 March 2022). "Calcium Glucarate: Purported Benefits, Side Effects & More". Retrieved 10 August 2025.
- ^ a b Ayyadurai, V. A. Shiva; Deonikar, Prabhakar; Fields, Christine (2023-02-01). "Mechanistic Understanding of D-Glucaric Acid to Support Liver Detoxification Essential to Muscle Health Using a Computational Systems Biology Approach". Nutrients. 15 (3): 733. doi:10.3390/nu15030733. ISSN 2072-6643. PMC 9921405. PMID 36771439.
- ^ Walaszek, Z. (October 1990). "Potential use of d-glucaric acid derivatives in cancer prevention". Cancer Letters. 54 (1–2): 1–8. doi:10.1016/0304-3835(90)90083-a. ISSN 0304-3835. PMID 2208084.
- ^ a b c Walaszek, Zbigniew; Hanausek-Walaszek, Malgorzata; Webb, Thomas E. (1984). "Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumorigenesis by 2,5-di-O-acetyl-D-glucaro-1, 4:6,3-dilactone, an in vivo β-glucuronidase inhibitor". Carcinogenesis. 5 (6): 767–772. doi:10.1093/carcin/5.6.767. ISSN 0143-3334. PMID 6202433.
- ^ a b Pasqualini, J.R.; Gelly, C. (April 1990). "Biological response of the anti-estrogen ICI 164,384 in human hormone-dependent and hormone-independent mammary cancer cell lines". Cancer Letters. 50 (2): 133–139. doi:10.1016/0304-3835(90)90243-q. ISSN 0304-3835. PMID 2328482.
- ^ a b c Crowell, Pamela L. (November 1997). "Monoterpenes in breast cancer chemoprevention". Breast Cancer Research and Treatment. 46 (2–3): 191–197. doi:10.1023/a:1005939806591. ISSN 0167-6806. PMID 9478274.
- ^ a b Whyte, Ian M. (January 2002). "Introduction: Research in Clinical Toxicology—The Value of High Quality Data". Journal of Toxicology: Clinical Toxicology. 40 (3): 211–212. doi:10.1081/clt-120005490. ISSN 0731-3810. PMID 12144193.