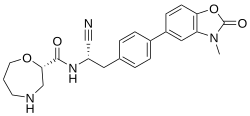
Brensocatib
 | |
| Clinical data | |
|---|---|
| Trade names | Brinsupri |
| Other names | AZD7986; INS1007 |
| AHFS/Drugs.com | Brinsupri |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Dipeptidyl peptidase 1 inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H24N4O4 |
| Molar mass | 420.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brensocatib, sold under the brand name Brinsupri, is a medication used for the treatment of bronchiectasis.[1] It is a dipeptidyl peptidase 1 inhibitor.[1][2] It is taken by mouth.[1]
Brensocatib was approved for medical use in the United States in August 2025.[1][3]
Medical uses
Brensocatib is indicated for the treatment of non-cystic fibrosis bronchiectasis in people aged twelve years of age and older.[1]
History
A phase III clinical trial, known as the ASPEN trial, was conducted to evaluate the safety and efficacy of brensocatib in patients with non-cystic fibrosis bronchiectasis.[4]
Society and culture
Legal status
Brensocatib was approved for medical use in the United States in August 2025.[5]
Names
Brensocatib is the international nonproprietary name.[6]
Brensocatib is sold under the brand name Brinsupri.[1]
References
- ^ a b c d e f g https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/217673s000lbl.pdf
- ^ Chalmers JD, Usansky H, Rubino CM, Teper A, Fernandez C, Zou J, et al. (October 2022). "Pharmacokinetic/Pharmacodynamic Evaluation of the Dipeptidyl Peptidase 1 Inhibitor Brensocatib for Non-cystic Fibrosis Bronchiectasis". Clinical Pharmacokinetics. 61 (10): 1457–1469. doi:10.1007/s40262-022-01147-w. PMC 9553789. PMID 35976570.
- ^ "Novel Drug Approvals for 2025". U.S. Food and Drug Administration (FDA). 15 August 2025. Retrieved 17 August 2025.
- ^ Chalmers JD, Burgel PR, Daley CL, De Soyza A, Haworth CS, Mauger D, et al. (April 2025). "Phase 3 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis". The New England Journal of Medicine. 392 (16): 1569–1581. doi:10.1056/NEJMoa2411664. PMID 40267423.
- ^ "FDA Approves Brinsupri (brensocatib) as the First and Only Treatment for Non-Cystic Fibrosis Bronchiectasis, a Serious, Chronic Lung Disease" (Press release). Insmed. 12 August 2025. Retrieved 17 August 2025 – via PR Newswire.
- ^ World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information. 34 (1). hdl:10665/339768.
External links
- Clinical trial number NCT04594369 for "A Study to Assess the Efficacy, Safety, and Tolerability of Brensocatib in Participants With Non-Cystic Fibrosis Bronchiectasis (ASPEN)" at ClinicalTrials.gov
- Clinical trial number NCT03218917 for "Assessment of INS1007 in Participants With Non-Cystic Fibrosis Bronchiectasis" at ClinicalTrials.gov