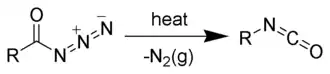Acyl azide
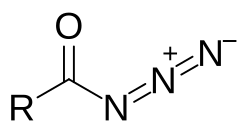
Acyl azides are carboxylic acid derivatives with the general formula RCON3. These compounds, which are a subclass of organic azides, are generally colorless.[1]
Preparation

Typically acyl azides are generated under conditions where they rearrange to the isocyanate.[1]
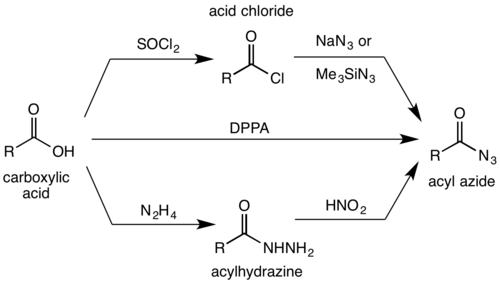
Acid chlorides[2][3] and anhydrides[4] react with sodium azide[5] or trimethylsilyl azide[6] to give acyl azides:
In a Mitsunobu variant, triphenylphosphine and trichloroacetonitrile catalyze excellent yields from various carboxylic acids and sodium azide at mild conditions.[7]
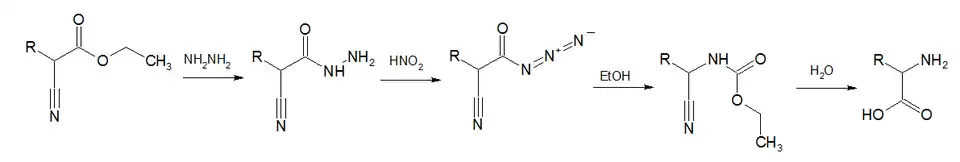
The second major route to azides is from treating acylhydrazines with nitrous acid.[1][8] Alternatively, the acyl azide can be formed by the direct reaction of a carboxylic acid with diphenylphosphoryl azide (DPPA).[9][10]
Another route oxidizes aldehydes with iodine azide, formed from sodium azide and iodine monochloride in acetonitrile.[11]
Uses
On Curtius rearrangement, acyl azides yield isocyanates.[12][13]
Acyl azides are also formed in Darapsky degradation,[14][15][16]
Historical references
- Curtius, Th. (1890). "Ueber Stickstoffwasserstoffsäure (Azoimid) N3H". Ber. (in German). 23 (2): 3023–3033. doi:10.1002/cber.189002302232.
- Curtius, Th. (1894). "20. Hydrazide und Azide organischer Säuren I. Abhandlung". J. Prakt. Chem. (in German). 50 (1): 275–294. doi:10.1002/prac.18940500125.
- Darapsky, August (1936). "Darstellung von α-Aminosäuren aus Alkyl-cyanessigsäuren". J. Prakt. Chem. (in German). 146 (8–12): 250–267. doi:10.1002/prac.19361460806.
- Darapsky, August; Hillers, Dietrich (1915). "Über das Hydrazid der Cyanessigsäure, Isonitrosocyanessigsäure und Nitrocyanessigsäure". J. Prakt. Chem. (in German). 92 (1): 297–341. doi:10.1002/prac.19150920117.
References
- ^ a b c Lwowski, Walter (1971). "Acyl azides". In Saul Patai (ed.). The Azido Group. PATAI'S Chemistry of Functional Groups. pp. 849–907. doi:10.1002/9780470771266.ch9. ISBN 9780470771679.
- ^ Allen, C. F. H.; Bell, Alan (1944). "Undecyl isocyanate". Organic Syntheses. 24: 94. doi:10.15227/orgsyn.024.0094.
- ^ Munch-Petersen, Jon (1953). "m-Nitrobenzazide (Benzoyl azide, m-nitro-)". Organic Syntheses. 33: 53. doi:10.15227/orgsyn.033.0053.
- ^ Weinstock, J (1961). "Modified Curtius reaction". J. Org. Chem. 26: 3511. doi:10.1021/jo01067a604.
- ^ Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry: Part B: Reactions and Synthesis (5th ed.). New York: Springer. p. 948. ISBN 978-0387683546.
- ^ Warren, J. D.; Press, J. B. (1980). "Formation and Curtius rearrangement of acyl azides from unreactive acid chlorides". Synth. Commun. 10: 107–110. doi:10.1080/00397918008061812.
- ^ Jang, Doo; Kim, Joong-Gon (2008). "Direct Synthesis of Acyl Azides from Carboxylic Acids by the Combination of Trichloroacetonitrile, Triphenylphosphine and Sodium Azide". Synlett. 2008 (13): 2072–2074. doi:10.1055/s-2008-1077979.
- ^ Pozsgay, V.; Jennings, H. J. (1987). "Azide synthesis with stable nitrosyl salts". Tetrahedron Lett. 28 (43): 5091–5092. doi:10.1016/s0040-4039(00)95598-9.
- ^ Shioiri, T.; Ninomiya, K.; Yamada, S. (1972). "New convenient reagent for a modified Curtius reaction and for peptide synthesis". J. Am. Chem. Soc. 94 (17): 6203–6205. doi:10.1021/ja00772a052. PMID 5054412.
- ^ Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry: Part B: Reactions and Synthesis (5th ed.). New York: Springer. p. 948. ISBN 978-0387683546.
- ^ Marinescu, Lavinia; Thinggaard, Jacob; Thomsen, Ib B.; Bols, Mikael (2003). "Radical Azidonation of Aldehydes". J. Org. Chem. 68 (24): 9453–9455. doi:10.1021/jo035163v. PMID 14629171.
- ^ Smith, Peter A. S. (1946). "The Curtius reaction". Org. React. 3: 337–449. doi:10.1002/0471264180.or003.09. ISBN 0471264180.
{{cite journal}}: ISBN / Date incompatibility (help) - ^ Scriven, Eric F. V.; Turnbull, Kenneth (1988). "Azides: Their preparation and synthetic uses". Chem. Rev. 88 (2): 297–368. doi:10.1021/cr00084a001.
- ^ Gagnon, Paul E.; Boivin, Paul A.; Craig, Hugh M. (1951). "Synthesis of Amino Acids from Substituted Cyanoacetic Esters". Can. J. Chem. 29 (1): 70–75. doi:10.1139/v51-009.
- ^ E. H. Rodd (1965). Chemistry of Carbon Compounds (2nd ed.). New York. p. 1157.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Gagnon, Paul E.; Nadeau, Guy; Côté, Raymond (1952). "Synthesis of α-Amino Acids from Ethyl Cyanoacetate". Can. J. Chem. 30 (8): 592–597. doi:10.1139/v52-071.