Actinidain
| actinidain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
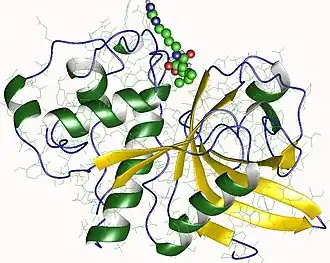 Biological assembly image of actinidain from Actinidia chinensis. From PDB: 1AEC. | |||||||||
| Identifiers | |||||||||
| EC no. | 3.4.22.14 | ||||||||
| CAS no. | 39279-27-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Actinidain (EC 3.4.22.14, actinidin, Actinidia anionic protease, proteinase A2 of Actinidia chinensis) is a type of cysteine protease enzyme found in fruits, including kiwifruit (genus Actinidia), pineapple, mango, banana, figs, and papaya. It is part of the peptidase C1 family of papain-like proteases.[1][2][3][4]
Actinidain is an allergen in kiwifruit.[5]
Actinidain is commercially used as a meat tenderizer[6][7] and in coagulating milk for dairy products, like yogurt and cheese.[8] The denaturation temperature of actinidain is 60 °C (140 °F), lower than that of similar meat tenderizing enzymes bromelain from pineapple and papain from papaya.[9]
History
Actinidain was first identified in 1959 when A.C. Arcus examined why jellies made with kiwifruit did not solidify,[10] an effect caused by a proteolytic enzyme acting on gelatin.[10] This enzyme was named actinidin as it was identified in a fruit of the genus Actinidia (Actinidia chinensis).[10] While similar proteins have been found in other fruits, this cysteine protease is unique to the kiwifruit.[10][11] A thiol group was identified to be essential for enzyme activity, which is why it was grouped with enzymes like papain and bromelain.[12][13]
Function
While no clear function has been identified, the enzyme begins to accumulate in the immature fruit and is suspected to be important for fruit development.[14] Actinidain has a detrimental effect on the larvae of Spodoptera litura, although its use as a pesticide has not been established.[11] It may also be used as a storage protein.[15]
Sequence and structure
Actinidain has an enzyme classification number (EC) of 3.4.22.14. The 3 classifies it as a hydrolase.[16] It is further classified as acting on peptide bonds, also known as a peptidase (3.4). The .22 represents the cysteine endopeptidases and then the .14 is actinidain’s unique identifier within that group.[16] Actinidain is first produced in the kiwifruit when it is about half its size and then increases in both protease activity and enzyme production until the fruit is fully matured.[11] The enzyme is encoded by a large gene family and is expressed in most tissues of the kiwifruit plant, not just the fruit itself.[11]
Actinidain is similar to papain in size, shape, active site location and conformation, as well as in kinetic studies, which is especially interesting as they only share 48% amino acid similarity.[2][12] Electron density mapping shows similar α-helices and overall polypeptide folding.[2][12] While the electron density map indicates 218 amino acids, further sequencing work suggests 220 amino acids with the extra two being found at the C-terminus.[12][13] The active site includes cysteine and histidine residues that are conserved across several other proteins in the fruit peptidase family.[13] Electron density mapping indicates a double crossover with domain 1 being made up of AA 19-115 and 214-218 and domain II composing of AA 1-18 and 116-213,[12] with both the N-terminal and the C-terminal ends crossing over into both domains. Domain 1 has several α-helices whereas domain 2 is primarily made up of one anti-parallel β-sheet.[12] Actinidain comprises up to 50% of the soluble protein content in kiwifruits at harvest.[17] Actinidain is active over a wide range of pH, including very acidic conditions,[18] with a pH optimum from 5-7.[19] At least ten different isoforms that all have the same molecular weight and cysteine protease activity as actinidain have been identified but they vary in isoelectric point from acidic (pI 3.9) to basic (pI 9.3).[17]
Allergic potential
Actinidain is the major allergen in kiwifruit, with the reaction presenting as mild symptoms in the mouth.[17][18]
Food applications
Actinidain is used as a high-quality meat tenderizer.[17] When marinating with pork, actinidain was found to tenderize it by affecting the myofibrils and connective tissue, which are similar to the tissues that are broken down through mechanical tenderization.[20][21]
Studies have shown that actinidain might be a good alternative milk coagulant, replacing chymosin, a common coagulant used in cheese making.[22]
References
- ^ Baker EN, Boland MJ, Calder PC, Hardman MJ (November 1980). "The specificity of actinidin and its relationship to the structure of the enzyme". Biochimica et Biophysica Acta (BBA) - Enzymology. 616 (1): 30–34. doi:10.1016/0005-2744(80)90260-0. PMID 7002215.
- ^ a b c Kamphuis IG, Drenth J, Baker EN (March 1985). "Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain". Journal of Molecular Biology. 182 (2): 317–329. doi:10.1016/0022-2836(85)90348-1. PMID 3889350.
- ^ Baker EN, Drenth J (1987). "The thiol proteases: structure and mechanism". In Jurnak FA, McPherson A (eds.). Active Sites of Enzymes. Biological Macromolecules and Assemblies. Vol. 3. New York: John Wiley and Sons. pp. 314–368. ISBN 978-0-471-85142-4.
- ^ Gul S, Mellor GW, Thomas EW, Brocklehurst K (May 2006). "Temperature-dependences of the kinetics of reactions of papain and actinidin with a series of reactivity probes differing in key molecular recognition features". The Biochemical Journal. 396 (1): 17–21. doi:10.1042/BJ20051501. PMC 1449998. PMID 16445383.
- ^ Maddumage R, Nieuwenhuizen NJ, Bulley SM, Cooney JM, Green SA, Atkinson RG (January 2013). "Diversity and relative levels of actinidin, kiwellin, and thaumatin-like allergens in 15 varieties of kiwifruit (Actinidia)". Journal of Agricultural and Food Chemistry. 61 (3): 728–739. Bibcode:2013JAFC...61..728M. doi:10.1021/jf304289f. PMID 23289429.
- ^ Bekhit AA, Hopkins DL, Geesink G, Bekhit AA, Franks P (2014). "Exogenous proteases for meat tenderization". Critical Reviews in Food Science and Nutrition. 54 (8): 1012–1031. doi:10.1080/10408398.2011.623247. PMID 24499119. S2CID 57554.
- ^ Eshamah H, Han I, Naas H, Acton J, Dawson P (April 2014). "Antibacterial effects of natural tenderizing enzymes on different strains of Escherichia coli O157:H7 and Listeria monocytogenes on beef". Meat Science. 96 (4): 1494–1500. doi:10.1016/j.meatsci.2013.12.010. PMID 24447905.
- ^ Katsaros GI, Tavantzis G, Taoukis PS (January 2010). "Production of novel dairy products using actinidin and high pressure as enzyme activity regulator". Innovative Food Science & Emerging Technologies. 11 (1): 47–51. doi:10.1016/j.ifset.2009.08.007.
- ^ Tarté R (2008). Ingredients in meat products properties, functionality and applications. New York: Springer. ISBN 978-0-387-71327-4.
- ^ a b c d Arcus AC (May 1959). "Proteolytic enzyme of Actinidia chinensis". Biochimica et Biophysica Acta. 33 (1): 242–244. doi:10.1016/0006-3002(59)90522-0. PMID 13651208.
- ^ a b c d Malone LA, Todd JH, Burgess EP, Philip BA, Christeller JT (June 2005). "Effects of kiwifruit (Actinidia deliciosa) cysteine protease on growth and survival of Spodoptera litura larvae (Lepidoptera: Noctuidae) fed with control or transgenic avidin-expressing tobacco". New Zealand Journal of Crop and Horticultural Science. 33 (2): 99–105. Bibcode:2005NZJCH..33...99M. doi:10.1080/01140671.2005.9514337. ISSN 0114-0671. S2CID 86179155.
- ^ a b c d e f Baker EN (September 1977). "Structure of actinidin: details of the polypeptide chain conformation and active site from an electron density map at 2-8 A resolution". Journal of Molecular Biology. 115 (3): 263–277. doi:10.1016/0022-2836(77)90154-1. PMID 592367.
- ^ a b c Carne A, Moore CH (July 1978). "The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis". The Biochemical Journal. 173 (1): 73–83. doi:10.1042/bj1730073. PMC 1185751. PMID 687380.
- ^ Praekelt UM, McKee RA, Smith H (May 1988). "Molecular analysis of actinidin, the cysteine proteinase of Actinidia chinensis". Plant Molecular Biology. 10 (3): 193–202. Bibcode:1988PMolB..10..193P. doi:10.1007/BF00027396. PMID 24277513. S2CID 21213015.
- ^ Chalabi M, Khademi F, Yarani R, Mostafaie A (April 2014). "Proteolytic activities of kiwifruit actinidin (Actinidia deliciosa cv. Hayward) on different fibrous and globular proteins: a comparative study of actinidin with papain". Applied Biochemistry and Biotechnology. 172 (8): 4025–4037. doi:10.1007/s12010-014-0812-7. PMID 24604128. S2CID 44438930.
- ^ a b "Information on EC 3.4.22.14 - actinidain". BRENDA Enzyme Database. Retrieved October 3, 2023.
- ^ a b c d Dearman RJ, Beresford L, Foster ES, McClain S, Kimber I (May 2014). "Characterization of the allergenic potential of proteins: an assessment of the kiwifruit allergen actinidin". Journal of Applied Toxicology. 34 (5): 489–497. doi:10.1002/jat.2897. PMID 23754484. S2CID 12609478.
- ^ a b Richardson DP, Ansell J, Drummond LN (December 2018). "The nutritional and health attributes of kiwifruit: a review". European Journal of Nutrition. 57 (8): 2659–2676. doi:10.1007/s00394-018-1627-z. PMC 6267416. PMID 29470689.
- ^ McDowall MA (June 1970). "Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme". European Journal of Biochemistry. 14 (2): 214–221. doi:10.1111/j.1432-1033.1970.tb00280.x. PMID 5506167.
- ^ Christensen M, Tørngren MA, Gunvig A, Rozlosnik N, Lametsch R, Karlsson AH, Ertbjerg P (July 2009). "Injection of marinade with actinidin increases tenderness of porcine M. biceps femoris and affects myofibrils and connective tissue". Journal of the Science of Food and Agriculture. 89 (9): 1607–1614. Bibcode:2009JSFA...89.1607C. doi:10.1002/jsfa.3633. ISSN 0022-5142.
- ^ Anaduaka EG, Chibuogwu CC, Ezugwu AL, Ezeorba TP (2023-04-03). "Nature-derived ingredients as sustainable alternatives for tenderizing meat and meat products: an updated review". Food Biotechnology. 37 (2): 136–165. doi:10.1080/08905436.2023.2201354. ISSN 0890-5436. S2CID 258559035.
- ^ Alirezaei M, Aminlari M, Gheisari HR, Tavana M (2011-03-22). "Actinidin: A Promising Milk Coagulating Enzyme". European Journal of Nutrition & Food Safety: 43–51. ISSN 2347-5641.
External links
- The MEROPS online database for peptidases and their inhibitors: C01.007
- EC 3.4.22.14
- actinidain at the U.S. National Library of Medicine Medical Subject Headings (MeSH)