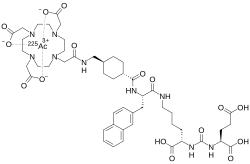
225Ac-PSMA-617
 | |
| Clinical data | |
|---|---|
| Other names | Actinium (225Ac) PSMA-617, UH4J18XEL3, Actinium vipivotide tetraxetan |
| Routes of administration | Intravenous |
| Drug class | Radiopharmaceutical |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C49H68AcN9O16 |
| 3D model (JSmol) | |
| |
| |
225Ac-PSMA-617 is an investigational radiopharmaceutical used in targeted alpha therapy (TAT) for metastatic castration-resistant prostate cancer (mCRPC) and, more recently, for metastatic hormone-sensitive prostate cancer (mHSPC). Initially developed by the German Cancer Research Center and University Hospital Heidelberg, it combines actinium-225 (225Ac), an alpha-emitting radionuclide, with PSMA-617, a small-molecule ligand that targets prostate-specific membrane antigen (PSMA), a protein overexpressed in prostate cancer cells. The therapy delivers high-energy alpha radiation to induce DNA damage in cancer cells while leveraging the short tissue penetration of alpha particles to minimize damage to healthy tissues. As of May 2025, 225Ac-PSMA-617 remains in early-phase clinical trials and has not yet received regulatory approval.[1][2]
Background
Prostate cancer is a leading cause of cancer-related mortality in men, with mCRPC representing an advanced, incurable stage resistant to androgen deprivation therapy (ADT). PSMA, a transmembrane protein, is highly expressed in prostate cancer cells, making it an ideal target for radioligand therapy. While lutetium-177 (177Lu)-PSMA-617 (approved as Pluvicto) has shown efficacy in mCRPC, some patients are resistant or develop diffuse bone marrow infiltration, limiting beta-emitter use due to hematologic toxicity. 225Ac-PSMA-617, an alpha-emitter, emerged to address these challenges, offering higher energy and shorter tissue penetration for precise tumor targeting. Initial clinical use began in 2014 as a salvage therapy under compassionate use protocols.[1][3]
Mechanism of action
225Ac-PSMA-617 targets PSMA on prostate cancer cells via the PSMA-617 ligand, which binds with high affinity and induces rapid cellular internalization. Actinium-225, with a 9.92-day half-life, emits four alpha particles in its decay chain, delivering high linear energy transfer (LET) radiation (100 keV/μm) that causes complex DNA double-strand breaks, leading to cancer cell death. The alpha particles' short range (47–85 μm) minimizes damage to surrounding tissues compared to beta-emitters like 177Lu. The DOTA chelator in PSMA-617 ensures stable 225Ac binding, enhancing tumor retention and reducing off-target effects.[4][1][5]
Clinical development
Clinical evaluation of 225Ac-PSMA-617 began with retrospective studies and compassionate use in mCRPC patients resistant to standard therapies. The AcTION trial (NCT04597411), a Phase I, open-label, dose-escalation study by Novartis, is assessing safety and tolerability in men with PSMA-positive mCRPC or mHSPC, with or without prior 177Lu-PSMA-617 exposure. Patients receive up to six cycles of 225Ac-PSMA-617 (100 kBq/kg) every 8 weeks, with 68Ga-PSMA-11 PET/CT confirming PSMA expression. Other trials, such as a Phase I study in China (2020–2021) and a Dutch trial with 225Ac-PSMA-I&T (NCT05902247), are exploring dosing and efficacy. As of May 2025, trials are ongoing, with no regulatory approvals.[2][6][7][8]
Combination strategies, including tandem therapy with 177Lu-PSMA-617 or immunotherapy, are being explored to enhance efficacy and reduce toxicity.
Efficacy
Early clinical data suggest 225Ac-PSMA-617 has significant anti-tumor activity. A 2016 study reported complete responses in two mCRPC patients, with prostate-specific antigen (PSA) levels dropping below detectable limits.[1] A 2020 prospective study of 28 mCRPC patients (54% post-177Lu-PSMA-617) showed a >50% PSA decline in 53.8–66.6% of patients, with median overall survival (OS) of 10–17 months.[6] A South African pilot study in 17 chemotherapy-naïve patients reported a ≥90% PSA decline in 82%, with 41% in remission at 12 months.[9] A 2022 meta-analysis found >80% of patients had some PSA decline, with 60% achieving >50% reduction.[10] Tandem therapy with 177Lu-PSMA-617 has shown promise in enhancing efficacy while reducing toxicity.[11]
Safety and tolerability
The primary dose-limiting toxicity of 225Ac-PSMA-617 is xerostomia (severe dry mouth) due to salivary gland uptake, affecting up to 25% of patients and leading to treatment discontinuation in some cases. Hematologic toxicities, including anemia, leukopenia, and thrombocytopenia, occur in up to one-third of patients, particularly those with extensive bone metastases. Renal toxicity is less common but reported in patients with pre-existing kidney issues. De-escalated dosing (e.g., 4–8 MBq) and tandem regimens with 177Lu-PSMA-617 have reduced salivary gland toxicity. Ongoing trials aim to optimize dosing to minimize adverse effects while maintaining efficacy.[6][9][11]
Challenges
The clinical adoption of 225Ac-PSMA-617 faces several hurdles. The global supply of 225Ac, primarily produced via thorium-229 decay or cyclotron methods, is limited, with annual production (e.g., 68 GBq from Karlsruhe, Oak Ridge, and Obninsk) supporting only thousands of doses.[12] Small sample sizes, retrospective designs, and heterogeneity in prior treatments limit current trial data, necessitating larger, randomized controlled trials. Salivary gland toxicity remains a significant barrier, with ongoing research into protective strategies like sialendoscopy.[10][7][13]
References
- ^ a b c d Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. (2016-12-01). "225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer". Journal of Nuclear Medicine. 57 (12): 1941–1944. doi:10.2967/jnumed.116.178673. PMID 27390158. Retrieved 2025-05-22.
- ^ a b "Study of 225Ac-PSMA-617 in Men With PSMA-positive Prostate Cancer". Novartis. 2025-03-21. Retrieved 2025-05-22.
- ^ Kairemo K, Kgatle M, Bruchertseifer F, Morgernstern A, Sathekge MM (2024). "Design of 225Ac-PSMA for targeted alpha therapy in prostate cancer". Annals of Translational Medicine. 12 (4): 67. doi:10.21037/atm-23-1842. PMC 11304416. PMID 39118950.
- ^ Ruigrok EA, Tamborino G, De Blois E, Roobol SJ, Verkaik N, De Saint-Hubert M, et al. (2021-08-02). "In vitro dose effect relationships of actinium-225- and lutetium-177-labeled PSMA-I&T". European Journal of Nuclear Medicine and Molecular Imaging. 49 (11): 3627–3638. doi:10.1007/s00259-022-05821-w. PMC 9399067. PMID 35556158.
- ^ Kairemo K, Kgatle M, Bruchertseifer F, Morgernstern A, Sathekge MM (August 2024). "Design of 225Ac-PSMA for targeted alpha therapy in prostate cancer". Annals of Translational Medicine. 12 (4): 67. doi:10.21037/atm-23-1842. PMC 11304416. PMID 39118950.
- ^ a b c Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C (2020-08-23). "Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients". Theranostics. 10 (20): 9364–9377. doi:10.7150/thno.48107. PMC 7415797. PMID 32802197.
- ^ a b Ling SW, van der Veldt AA, Konijnenberg M, Segbers M, Hooijman E, Bruchertseifer F, et al. (2024-01-29). "Evaluation of the tolerability and safety of [225Ac]Ac-PSMA-I&T in patients with metastatic prostate cancer: a phase I dose escalation study". BMC Cancer. 24 (1) 146. doi:10.1186/s12885-024-11900-y. PMC 10826262. PMID 38287346.
- ^ Endocyte (2025-03-20). AcTION: A Phase I Study of [225Ac]Ac-PSMA-617 in Men With PSMA-positive Prostate Cancer With or Without Prior [177Lu]Lu-PSMA-617 Radioligand Therapy (Report). clinicaltrials.gov.
- ^ a b Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. (2018-09-19). "225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study". European Journal of Nuclear Medicine and Molecular Imaging. 46 (1): 129–138. doi:10.1007/s00259-018-4167-0. PMC 6267694. PMID 30232539.
- ^ a b Ma J, Li L, Liao T, Gong W, Zhang C (2022). "Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis". Frontiers in Oncology. 12: 796657. doi:10.3389/fonc.2022.796657. PMC 8852230. PMID 35186737.
- ^ a b "Ac-225-PSMA-617". Oncidium Foundation. 2021-09-10. Retrieved 2025-05-22.
- ^ "Multiple Production Methods Underway to Provide Actinium-225". National Isotope Development Center. 2022-04-05. Retrieved 2025-05-22.
- ^ Kratochwil C, Haberkorn U, Giesel FL (2020). "225Ac-PSMA-617 for Therapy of Prostate Cancer". Seminars in Nuclear Medicine. 50 (2): 133–140. doi:10.1053/j.semnuclmed.2020.02.004. PMID 32172798.