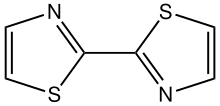
2,2'-Bithiazole
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H4N2S2 | |
| Molar mass | 168.23 g·mol−1 |
| Appearance | white solid |
| Density | 1.82 g/cm3 |
| Melting point | 102.5 °C (216.5 °F; 375.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
2,2'-Bithiazole is an organic compound with the formula (H2C3NS)2. The molecule consists of two thiazole rings linked by a C-C bond. Diverse isomers are possible depending on the carbon atoms that are coupled, but the 2,2' isomer is common. The compound was first prepared by Ullmann coupling of 2-bromothiazole using copper metal.[1]
2,2'-Bithiazole is planar, according to X-ray crystallography.[2]
Occurrence
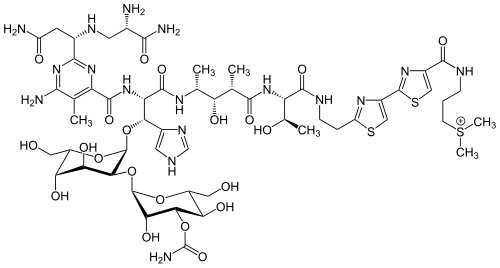
2,2'-Bithiazole itself is mainly of academic interest, but substituted, isomeric bithiazoles have attracted attention in medicinal chemistry. Naturally occurring bithiazoles are derived from cysteine, as are most naturally occurring thiazoles. Well-studied derivatives are the bleomycins, which feature 2,4'-bithiazole incorporated into glycopeptides. The cystothiazoles are another family of bithiazoles, but they feature 2,5-linkage. Luciferin contains a benzothiazole subunit linked to a thiazolidine (dihydrothiazole) via a 2,2' linkage.[3]
2,2'-Bithiazole forms a variety of coordination complexes.[2]
References
- ^ Erlenmeyer, H.; Schmid, Erich H. (1939). "Über isostere und strukturähnliche Verbindungen XI. Darstellung und Eigenschaften des 2,2′-Dithiazolyl". Helvetica Chimica Acta. 22: 698–700. doi:10.1002/hlca.19390220186.
- ^ a b Craig, DC; Goodwin, H. A.; Onggo, D.; Rae, AD (1988). "Coordination of 2,2'-Bithiazole. Spectral, Magnetic and Structural Studies of the Iron(II) and Nickel(II) Complexes". Australian Journal of Chemistry. 41 (11): 1625. doi:10.1071/CH9881625.
- ^ Jin, Zhong (2011). "Muscarine, imidazole, oxazole, and thiazole alkaloids". Natural Product Reports. 28 (6): 1143–1191. doi:10.1039/C0NP00074D. PMID 21472175.