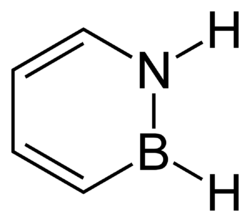
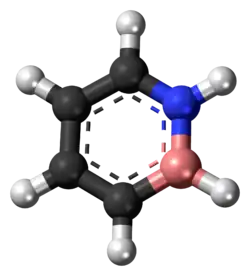
1,2-Dihydro-1,2-azaborine
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Dihydro-1,2-azaborine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H6BN | |||
| Molar mass | 78.908 g mol−1 | ||
| Appearance | clear, colorless liquid | ||
| Melting point | −46 to −45 °C. | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
1,2-Dihydro-1,2-azaborine is an aromatic chemical compound with properties intermediate between benzene and borazine. Its chemical formula is C4BNH6. It resembles a benzene ring, except that two adjacent carbons are replaced by nitrogen and boron, respectively. Several other 1,2-azaborines have been synthesized.[1]
Preparation
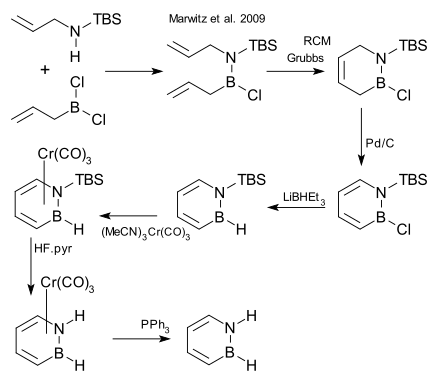
After decades of failed attempts, the compound was synthesized in 2008 and reported in January 2009.[2][3]
One of the synthetic steps is a ring-closing metathesis (RCM) reaction:[4]
References
- ^ Dewar, Michael J. S.; Marr, Peter A. (1962-10-01). "A Derivative of Borazarene". Journal of the American Chemical Society. 84 (19): 3782–3782. doi:10.1021/ja00878a045. ISSN 0002-7863.
- ^ Stu Borman. "Long-Sought Benzenelike Molecule Created: Aromaticity of organic-inorganic hybrid resembles benzene's." C&EN January 5, 2009 Volume 87, Number 01 p. 11
- ^ A. J. V. Marwitz; M. H. Matus; L. N. Zakharov; D. A. Dixon; S.-Y. Liu (January 2009). "A Hybrid Organic/Inorganic Benzene". Angew. Chem. Int. Ed. 48 (5): 973–977. doi:10.1002/anie.200805554. PMID 19105174.
- ^ TBS = tert-butyldimethylsilyl, step 2 RCM = ring-closing metathesis using Grubbs' catalyst, step 3 organic oxidation using palladium on carbon, step 4 reduction LiBHEt3, step 5 conversion to piano stool complex as protective group with chromium carbonyl derivative, step 6 cleavage N-TBS bond HF, step 7 deprotection with triphenylphosphine